博文
生物芯片股票跌;分子诊断唱主角。
 精选
精选
|||
前两天刚讲到药物基因组代表公司Perlegene关门,今天又看到生物芯片旗舰公司Affymetrix股价大跌。跌价是因为华尔街股票分析师们投了“不信任票”,认为新一代高通量测序技术已经或正在取代芯片技术。下面是Affymetrix公司的历年股价走势:
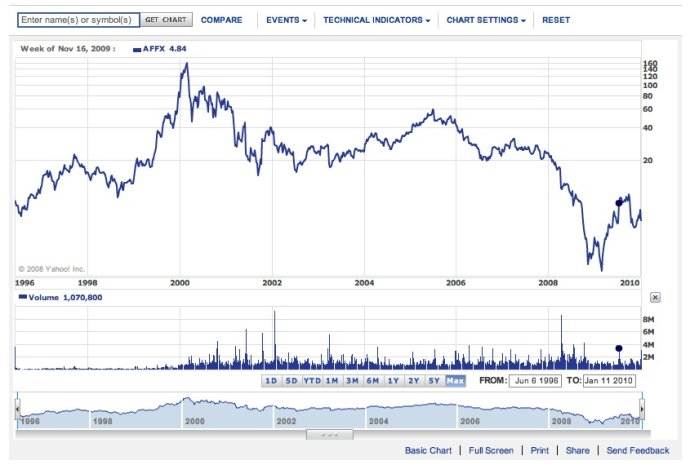
2000年的时候高到$160美元,今天$5.53!! 我2000年的时候还买了不少他们的股票,结果几乎全赔进去了,到$20-$30的时候卖的。从那以后我自己就不买股票了,伤透了心。
不过,Affy还有机会起死回生。他们还有每年三亿美金的收入,银行里还有快三亿的现金,不过还欠有两亿多的债。能否迅速找到新的增长点是他们成功的关键。
Affy以原位合成核酸芯片技术称霸一时。但是以杂交为原理的核酸识别技术毕竟不如各种高通量测序技术来的精细,更方便,数据更多,更直接,也更便宜。 Affy的经营情形给整个生物芯片行业敲响了警钟。也给所有生物技术公司敲响了警钟。这个警钟就是要我们居安思危,在发展势头上的时候就要考虑到新技术可能对我们的冲击:优势永远是相对的,竞争永远是绝对的。
从技术开发者的角度看,要认识到竞争优势的短暂性;从技术使用者的角度看,要尽早使用最新的仪器才能永远站在行业的前面。
做公司的,关心市场情报也是必修课之一。别的公司都在做什么?有什么打算?每年一月份在旧金山开这个JP Morgan Healthcare Conference 就是收集这类情报的最佳时机。这一般是上市公司展望新的一年的“表决心”的会。有点象传说中的武林大会:一般都是公司CEO来向投资者说他们今年的打算,有什么新产品上市。历年都是以药物和医疗器械为主,今年分子诊断占了显著的位置。Still, nobody has what we have to offer!! It is up to us to deliver. 也许明年这个会上就能听到我们iCubate的台词了:Multiplex amplification and detection, automatically, in a disposable and closed system. 下面文章是介绍我熟悉的几个公司(Qiagen, Cephied, Luminex, Gen-Probe等)今年的打算。
Qiagen 有新仪器上市,并有配套的试剂;Cephied主要是充实试剂;Luminex也没有什么新花样。看看这个竞争态势,好像美式足球一样挤在一起,不过我看到了中间的一条逢刚好我们能钻过去,冲到底线!这条路就是多重扩增,全自动,封闭的分子诊断技术平台。
看看这些分子诊断公司的技术路线,产品种类,市场规模和做市场的方式方法,真的就象是在“华山论剑”武场上偷学武艺一样。如果没有创业经历,我可能对这类信息不会产生任何兴趣。学会观察市场才知道自己的差距在那里,优势在那里。每次参加专业会议,我花在展区的时间要比在会场上听讲的时间多。不但可以获得一手的商品和技术信息,更能有机会和其它创业者交流,得到宝贵的创业经验。
下面是GenomeWeb记者写的稿子:
****
JP Morgan Healthcare Conference: MDx Firms See Major Launches in '10-'11
January 14, 2010
By Ed Winnick
SAN FRANCISCO (GenomeWeb News) - Several molecular diagnostic firms are looking to unveil new assays and instrument systems that could greatly expand their market presence, company executives told investors at the JP Morgan Healthcare Conference held here this week.
Qiagen, which currently has a molecular diagnostic assay portfolio of around 120 tests, is planning to launch news instruments over the next couple of years targeting the entire molecular diagnostics market, from point of care to high-throughput labs.
The firm will soon be rolling out in Europe its QIAensemble high-throughput system, which will have assays for human papillomavirus, chlamydia, gonorrhea, and others. Qiagen hopes that following a 2010 launch in Europe, the system will make its way to the US market in 2012, according to a presentation given by CEO Peer Schatz on Wednesday.
The firm also is targeting the pharmacogenomics market with the launch of the QIAsymphony platform in Europe this year followed by the US in 2011. Schatz said that the company has more than 15 partnerships with pharmaceutical firms to develop pharmacogenomic or companion diagnostic assays. Among those partners are Amgen, Eli Lilly, Merck, ImClone, Boehringer Ingelheim, and Bristol-Myers Squibb.
He said Qiagen intends to file pre-market applications for its KRAS test, for which it has partnered with Amgen, and its EGFR test, in the next two years. Other PGx assays are planned for 2011 to 2014.
Schatz said that when the QIAsymphony is launched it will mark the largest molecular diagnostic offering in the world.
While the firm recently raised its profile in the personalized diagnostics field through its acquisition of DxS, Schatz told GenomeWeb Daily News that Qiagen already does around $50 million in business in the field, and has many partnerships with pharma firms that have not been publicly disclosed.
Also on the way from Qiagen is a point-of-care, portable molecular diagnostics instrument capable of running eight samples at a time. The small machine weighs only half a pound and can run on 3 AA batteries. Schatz said that the instrument would first be deployed in applied markets, with acute care and mobile care being key markets as well. In addition, he said that developing tests for healthcare-associated infections for the instrument are an option.
The instrument was acquired along with its maker, Germany's ESE, a privately held developer and manufacturer of UV and fluorescence optical measurement devices, for $19 million in cash this week.
Overall, the next 12 to 18 months are going to be a "big submission window" as far as regulatory filings go, Schatz told GWDN.
Cepheid
Another firm that is looking to broaden its assay menu is Cepheid, which has been known primarily for its portfolio of HAI tests. John Bishop, the firm's CEO, told investors today that the company's 2010 milestones include the clearance and launch of six tests, two of which — one for flu, and one for VanA, an antimicrobial resistance gene most commonly associated with vancomycin-resistant enterococci — have already been cleared for marketing.
The firm has introductions planned for an MRSA/SA nasal test, a Flu A/B panel, a C. difficile/Epi test, and is targeting the European release of a chlamydia/gonorrhea test this year.
Unlike Qiagen, Bishop believes the company's strategy of trying to serve the market with one scalable platform from low- to high-throughput is an advantage. According to Bishop, labs want to consolidate on a few platforms with a wide menu offered for those systems.
He also said that the firm's newest platform, the Infinity 48, is one or two generations ahead of competitors.
Also new for Cepheid is a development program for a microRNA-based test for lung cancer. Bishop believes that it is possible that the potential test, which he hopes will be based on multiple markers, will eliminate the need for biopsies, as the key marker in development has been detected in serum in both early- and late-stage lung cancers.
Gen-Probe
Gen-Probe will launch its new molecular diagnostics instrument, called Panther, this year, President and CEO Carl Hull said at the conference on Wednesday. The new system will target low-to-mid-volume labs. It will be fully automated, have a smaller footprint, and offer random access testing of qualitative and quantitative assays, which Hull said will enable molecular diagnostic testing capabilities not currently available on its Tigris system.
Assays that run on Panther initially will be similar to the ones on Tigris for chlamydia and gonorrhea. There also are plans for a human papillomavirus assay and its Progensa PCA3 assay to help physicians identify men at risk of increased risk of prostate cancer.
Among the firm's 2010 priorities is to launch Panther in Europe, with a US launch planned for four or five quarters later. Gen-Probe also plans to file for FDA clearance of its Aptima HPV for the Tigris system and an Aptima trichomonas assay for identifying the STD trichomoniasis. It also will continue developing Panther for blood screening, with funding for that project coming from its partner Novartis.
Hull also fired a shot across the bow of other molecular diagnostic firms, saying that the firm's Tigris system has attracted "imitators," and saying that companies "need to be mindful" of Gen-Probe's patent estate. He suggested that the company could license IP to some parties, but would not comment on whether the firm would be pursuing legal action against any competitors in the near future.
Celera
Celera CEO Kathy Ordonez told investors that the company is in the midst of hiring 23 new sales representatives to detail its genetic tests. Thus far, it has hired 17 of those reps.
The firm is trying to expand the market for its KIF6 test, which tests for cardiovascular risk and statin benefit and is currently offered through Celera's Berkeley Heart Labs business. Among those efforts is a collaboration with Medco to study the ability of KIF6 testing to help in getting patients to take their statins. Earlier this week, the firm also licensed rights to the University of California, San Francisco to develop an in-house test for the gene.
Ordonez also said that the firm is discussing with the US Food and Drug Administration the filing of a pre-market approval application for the KIF6 test. The company expects to submit that application in the second half of 2010.
Luminex
Luminex President and CEO Patrick Balthrop noted that the firm expects full-year 2009 revenues of between $119 million and $121 million, revised from its projected revenue guidance in November of between $118 and $126 million.
He also told investors that the firm is aiming to introduce several new products in 2010, including its RVP Fast testing panel, which has been launched in Europe but will be filed for clearance in the US soon. Its test pipeline includes ag/bio and gastrointestinal panels that the firm hopes to launch in 2010.
Luminex also intends to launch its MagPix instrument platform in the second half of the year, expanding the firm's reach into the low-throughput market, said Balthrop. In late 2008, Luminex launched its FlexMAP 3D, a higher-throughput, higher-cost system aimed at larger labs.
https://blog.sciencenet.cn/blog-290052-287346.html
上一篇:月底去生物物理所交流创业经历
下一篇:到处都有创新的机会

2000年的时候高到$160美元,今天$5.53!! 我2000年的时候还买了不少他们的股票,结果几乎全赔进去了,到$20-$30的时候卖的。从那以后我自己就不买股票了,伤透了心。
不过,Affy还有机会起死回生。他们还有每年三亿美金的收入,银行里还有快三亿的现金,不过还欠有两亿多的债。能否迅速找到新的增长点是他们成功的关键。
Affy以原位合成核酸芯片技术称霸一时。但是以杂交为原理的核酸识别技术毕竟不如各种高通量测序技术来的精细,更方便,数据更多,更直接,也更便宜。 Affy的经营情形给整个生物芯片行业敲响了警钟。也给所有生物技术公司敲响了警钟。这个警钟就是要我们居安思危,在发展势头上的时候就要考虑到新技术可能对我们的冲击:优势永远是相对的,竞争永远是绝对的。
从技术开发者的角度看,要认识到竞争优势的短暂性;从技术使用者的角度看,要尽早使用最新的仪器才能永远站在行业的前面。
做公司的,关心市场情报也是必修课之一。别的公司都在做什么?有什么打算?每年一月份在旧金山开这个JP Morgan Healthcare Conference 就是收集这类情报的最佳时机。这一般是上市公司展望新的一年的“表决心”的会。有点象传说中的武林大会:一般都是公司CEO来向投资者说他们今年的打算,有什么新产品上市。历年都是以药物和医疗器械为主,今年分子诊断占了显著的位置。Still, nobody has what we have to offer!! It is up to us to deliver. 也许明年这个会上就能听到我们iCubate的台词了:Multiplex amplification and detection, automatically, in a disposable and closed system. 下面文章是介绍我熟悉的几个公司(Qiagen, Cephied, Luminex, Gen-Probe等)今年的打算。
Qiagen 有新仪器上市,并有配套的试剂;Cephied主要是充实试剂;Luminex也没有什么新花样。看看这个竞争态势,好像美式足球一样挤在一起,不过我看到了中间的一条逢刚好我们能钻过去,冲到底线!这条路就是多重扩增,全自动,封闭的分子诊断技术平台。
看看这些分子诊断公司的技术路线,产品种类,市场规模和做市场的方式方法,真的就象是在“华山论剑”武场上偷学武艺一样。如果没有创业经历,我可能对这类信息不会产生任何兴趣。学会观察市场才知道自己的差距在那里,优势在那里。每次参加专业会议,我花在展区的时间要比在会场上听讲的时间多。不但可以获得一手的商品和技术信息,更能有机会和其它创业者交流,得到宝贵的创业经验。
下面是GenomeWeb记者写的稿子:
****
JP Morgan Healthcare Conference: MDx Firms See Major Launches in '10-'11
January 14, 2010
By Ed Winnick
SAN FRANCISCO (GenomeWeb News) - Several molecular diagnostic firms are looking to unveil new assays and instrument systems that could greatly expand their market presence, company executives told investors at the JP Morgan Healthcare Conference held here this week.
Qiagen, which currently has a molecular diagnostic assay portfolio of around 120 tests, is planning to launch news instruments over the next couple of years targeting the entire molecular diagnostics market, from point of care to high-throughput labs.
The firm will soon be rolling out in Europe its QIAensemble high-throughput system, which will have assays for human papillomavirus, chlamydia, gonorrhea, and others. Qiagen hopes that following a 2010 launch in Europe, the system will make its way to the US market in 2012, according to a presentation given by CEO Peer Schatz on Wednesday.
The firm also is targeting the pharmacogenomics market with the launch of the QIAsymphony platform in Europe this year followed by the US in 2011. Schatz said that the company has more than 15 partnerships with pharmaceutical firms to develop pharmacogenomic or companion diagnostic assays. Among those partners are Amgen, Eli Lilly, Merck, ImClone, Boehringer Ingelheim, and Bristol-Myers Squibb.
He said Qiagen intends to file pre-market applications for its KRAS test, for which it has partnered with Amgen, and its EGFR test, in the next two years. Other PGx assays are planned for 2011 to 2014.
Schatz said that when the QIAsymphony is launched it will mark the largest molecular diagnostic offering in the world.
While the firm recently raised its profile in the personalized diagnostics field through its acquisition of DxS, Schatz told GenomeWeb Daily News that Qiagen already does around $50 million in business in the field, and has many partnerships with pharma firms that have not been publicly disclosed.
Also on the way from Qiagen is a point-of-care, portable molecular diagnostics instrument capable of running eight samples at a time. The small machine weighs only half a pound and can run on 3 AA batteries. Schatz said that the instrument would first be deployed in applied markets, with acute care and mobile care being key markets as well. In addition, he said that developing tests for healthcare-associated infections for the instrument are an option.
The instrument was acquired along with its maker, Germany's ESE, a privately held developer and manufacturer of UV and fluorescence optical measurement devices, for $19 million in cash this week.
Overall, the next 12 to 18 months are going to be a "big submission window" as far as regulatory filings go, Schatz told GWDN.
Cepheid
Another firm that is looking to broaden its assay menu is Cepheid, which has been known primarily for its portfolio of HAI tests. John Bishop, the firm's CEO, told investors today that the company's 2010 milestones include the clearance and launch of six tests, two of which — one for flu, and one for VanA, an antimicrobial resistance gene most commonly associated with vancomycin-resistant enterococci — have already been cleared for marketing.
The firm has introductions planned for an MRSA/SA nasal test, a Flu A/B panel, a C. difficile/Epi test, and is targeting the European release of a chlamydia/gonorrhea test this year.
Unlike Qiagen, Bishop believes the company's strategy of trying to serve the market with one scalable platform from low- to high-throughput is an advantage. According to Bishop, labs want to consolidate on a few platforms with a wide menu offered for those systems.
He also said that the firm's newest platform, the Infinity 48, is one or two generations ahead of competitors.
Also new for Cepheid is a development program for a microRNA-based test for lung cancer. Bishop believes that it is possible that the potential test, which he hopes will be based on multiple markers, will eliminate the need for biopsies, as the key marker in development has been detected in serum in both early- and late-stage lung cancers.
Gen-Probe
Gen-Probe will launch its new molecular diagnostics instrument, called Panther, this year, President and CEO Carl Hull said at the conference on Wednesday. The new system will target low-to-mid-volume labs. It will be fully automated, have a smaller footprint, and offer random access testing of qualitative and quantitative assays, which Hull said will enable molecular diagnostic testing capabilities not currently available on its Tigris system.
Assays that run on Panther initially will be similar to the ones on Tigris for chlamydia and gonorrhea. There also are plans for a human papillomavirus assay and its Progensa PCA3 assay to help physicians identify men at risk of increased risk of prostate cancer.
Among the firm's 2010 priorities is to launch Panther in Europe, with a US launch planned for four or five quarters later. Gen-Probe also plans to file for FDA clearance of its Aptima HPV for the Tigris system and an Aptima trichomonas assay for identifying the STD trichomoniasis. It also will continue developing Panther for blood screening, with funding for that project coming from its partner Novartis.
Hull also fired a shot across the bow of other molecular diagnostic firms, saying that the firm's Tigris system has attracted "imitators," and saying that companies "need to be mindful" of Gen-Probe's patent estate. He suggested that the company could license IP to some parties, but would not comment on whether the firm would be pursuing legal action against any competitors in the near future.
Celera
Celera CEO Kathy Ordonez told investors that the company is in the midst of hiring 23 new sales representatives to detail its genetic tests. Thus far, it has hired 17 of those reps.
The firm is trying to expand the market for its KIF6 test, which tests for cardiovascular risk and statin benefit and is currently offered through Celera's Berkeley Heart Labs business. Among those efforts is a collaboration with Medco to study the ability of KIF6 testing to help in getting patients to take their statins. Earlier this week, the firm also licensed rights to the University of California, San Francisco to develop an in-house test for the gene.
Ordonez also said that the firm is discussing with the US Food and Drug Administration the filing of a pre-market approval application for the KIF6 test. The company expects to submit that application in the second half of 2010.
Luminex
Luminex President and CEO Patrick Balthrop noted that the firm expects full-year 2009 revenues of between $119 million and $121 million, revised from its projected revenue guidance in November of between $118 and $126 million.
He also told investors that the firm is aiming to introduce several new products in 2010, including its RVP Fast testing panel, which has been launched in Europe but will be filed for clearance in the US soon. Its test pipeline includes ag/bio and gastrointestinal panels that the firm hopes to launch in 2010.
Luminex also intends to launch its MagPix instrument platform in the second half of the year, expanding the firm's reach into the low-throughput market, said Balthrop. In late 2008, Luminex launched its FlexMAP 3D, a higher-throughput, higher-cost system aimed at larger labs.
https://blog.sciencenet.cn/blog-290052-287346.html
上一篇:月底去生物物理所交流创业经历
下一篇:到处都有创新的机会
扫一扫,分享此博文
全部作者的精选博文
全部作者的其他最新博文
全部精选博文导读
相关博文
- • Minerals线下恳谈会:履践致远、与时偕行——对话中国科学院广州地球化学研究所期刊合作学者
- • 聚英才 建高地 | 北京理工大学“特立青年学者”全球招聘开启
- • 700年后日本或濒临灭绝?日本学者推算预测:届时或仅剩1名15岁以下孩子
- • [转载]【同位素视角】非英语母语学者如何区分’e.g.’, ‘i.e.’, ‘namely’与‘such as’等混淆难题
- • 美国佐治亚大学等机构学者:刈割策略对Bulldog 805紫花苜蓿+Tifton 85狗牙根混播草地产量及品质的影响
- • 美国堪萨斯州立大学、密苏里大学等机构学者研究成果:土壤水分管理策略和品种多样性对紫花苜蓿产量、营养品质和农场盈利能力的影