博文
金属氢再谈:Thanks to Neil Ashcroft and Fukai
||
关注:
1) 金属氢与物理学家Neil Ashcroft的
2) 氢化物的最新及经典文献收集 解读
FeHn(1-3)
FeH2 I4/mmm. a= 2.479 Å, c =8.415 Å, Z = 4 at 67 GPa
The structure, obtained at 67 GPa by placing 8 H atoms in the 4c (0, 1/2, 0) and 4d (0, 1/2, 1/4) positions, Fe 4e (0, 0, 0.853).
FeH P63/mmc a= 2.645 Å, c =8.67 Å, Z =4 at 64 GPa
Fe:2a(0 0 0) 2c (1/3,2/3,1/4) H:4f(1/3,2/3,0.880)
FeH3 Pm-3m a= 2.437 Å, Z = 1 at 87 GPa
Fe 1a (0, 0, 0) H 3c (0, 1/2, 1/2)
摘录学习:
http://hoffmann.chem.cornell.edu/guoying-gao/
I am very interested in the crystal structure, chemical bonding, phase transition, electronic structure, lattice dynamics and superconductivity of condensed matter under high pressure. I am concentrated on the following two projects:
1. Nb-H system
Searching for metallic hydrogen has been the topic for more than a half century. However, the realization of metallic hydrogen within the reach of diamond anveil cell still remains unsolved.
In 1983, Carlsson and Ashcroft proposed that impurities could induce the insulator-metal transition in solid hydrogen at a lower external pressure required. They suggested that transition metals might be good choice due to that transition metals are known to effectively dissociate hydrogen molecules and some transition metals has formed hydrogen-rich compounds, such as YH3.
We choose Nb as an example due to that pure Nb holds the record for the highest superconducting transition temperature Tc (9.3 K) of an element at normal pressure; And pure H2 itself is predicted to be a very good high-temperature superconductor due to its very high phonon frequency, if it could be metalized.
So how about if we put a little bit of Nb into the dense hydrogen?
文献解读:
To what extent hydrides with a highH:metal ratio are analogous to metal hydrogen is ofgreat fundamental interest.
At 110 GPa, the H-H nearest distance is1.70 Å in FeH3, significantly larger than the 1.54 Å value in AlH3[35] but shorter than the 1.84 Å value inIrH3 [17].
In these hydrides, the H-H distancediminishes【减少】 slowly with pressure, going to 1.66 Å inFeH3 at 160 GPa. To reach the 1.0 Å H-Hdistance expected for metal hydrogen at 450 GPa [36] would require pressures of∼850 GPa.
On the other hand, the layer of atomic Hin FeH2 or the H sublattice in FeH3 could have interestingproperties analogous to those of expanded metal hydrogen. In addition, FeH3, nonferromagnetic above 40 GPa, is calculatedto be metallic: it would be very interesting to investigate if it is a superconductor as it has been proposed for hydrogen dominant alloys [11].
【36】J. M. McMahon and D. M. Ceperley, Phys. Rev. Lett. 106, 165302 (2011).
【11】N.W. Ashcroft, Phys. Rev. Lett. 92, 187002 (2004).
http://wjst.wu.ac.th/index.php/wjst/article/view/510
The electronic structure and structural phase transition of TiHn (n = 1, 2 and 3) are investigated using the Tight-Binding Linear Muffin-Tin Orbital (TB-LMTO) method with Local density approximation (LDA) and Atomic sphere approximation (ASA). The equilibrium geometries, the electronic band structure, and the total and partial Density of States (DOS) are obtained under various pressures, and are analyzed in comparison with the available experimental and theoretical data. It is predicted that the most stable structure of Titanium hydride is a cubic structure at normal pressure. Both TiH and TiH2 undergo a structural phase transition from a cubic to a hexagonal phase under high pressure. The stability of TiM2H and TiMH2 is analyzed.
2. Site occupancy of interstitial deuterium atoms in face-centred cubic iron
http://www.nature.com/ncomms/2014/140926/ncomms6063/abs/ncomms6063.html
Hydrogen composition and occupation state provide basic information for understanding various properties of the metal–hydrogen system, ranging from microscopic properties such as hydrogen diffusion to macroscopic properties such as phase stability.
Here the deuterization process of face-centred cubic Fe to form solid-solution face-centred cubic FeDx is investigated using in situ neutron diffraction at high temperature and pressure.
In a completely deuterized specimen at 988 K and 6.3 GPa, deuterium atoms occupy octahedral and tetrahedral interstitial sites with an occupancy of 0.532(9) and 0.056(5), respectively, giving a deuterium composition x of 0.64
(1).【氢在不锈钢中的渗透扩散】
During deuterization, the metal lattice expands approximately linearly with deuterium composition at a rate of 2.21 Å3 per deuterium atom.
The minor occupation of the tetrahedral site is thermally driven by the intersite 【格点之间】movement of deuterium atoms along the ‹111› direction in the face-centred cubic metal lattice.
Site occupancy of H atom is also essential for the characterization of fcc FeHx.
The fcc metal lattice has two interstitial sites available for accommodating H atoms: octahedral (O) and tetrahedral (T) sites.
In transition metals with fcc lattice, dissolved H atoms preferentially occupy the O site with larger free space than the T site10.
T-site occupation has been reported only for fcc PdDx and seems to remain inconclusive11.
Volume expansion vH caused by hydrogen absorption is another essential quantity; a value of 2.4 Å3 per H4 has been reported for dhcp-FeH, which is significantly larger than those of 1.66–1.82 Å3 per H for other 3d-transition metals’ hydrides with hcp metal lattice10.
For the vH of fcc FeHx, an experimentally determined value has not been reported; the value of 1.9 Å3 per H conventionally used for fcc FeHx was derived from the concentration of hydrogen measured in fcc-(Fe0.65Mn0.29Ni0.06)H0.95, which was cooled to ~220 K (ref. 12). Recently, a slightly larger value (2.09 Å3 per H), obtained from the compression data of fcc FeHx, has been proposed2, 7, 8.
经典解读:Springer2005-The metal-hydrogen system-by Fukai
http://www.springer.com/materials/book/978-3-540-00494-3
(1) 1s-like orbitals around the proton hybridized
with d-orbitals of surrounding M-atoms
spacing between neighboring tetrahedral sites
When a hydrogen atom is placed in a metallic environment, conduction
electrons screen the proton charge at short distances to make it appear as
a neutral atom.
Electronic calculations performed for hydrogen in transition
metals have shown that 1s-like orbitals around the proton are strongly hybridized
with d-orbitals of surrounding M-atoms to form bonding states (see
Chap. 7).
Thus, the apparent small size of an H atom, that allows it to enter
narrow interstitial sites in metal lattices, is due in part to the flexibility of
surrounding electronic states. A small positive charge on a proton leaves the
surrounding electrons easily adaptable to given circumstances.
These estimates of the spatial extension of the wave function immediately
point to the possibility of tunneling of an H atom between interstitial sites.
In bcc metals, in which the spacing between neighboring tetrahedral sites is
∼1.0˚A, evidence of tunneling processes was indeed observed.
(2) Metallic hydrides,interstitial alloys,a variety of phases can exist in M–H binary alloys.
Metallic hydrides, by nature of metallic bonding, commonly exist over
extended ranges of nonstoichiometric compositions.
These hydrides can be
called interstitial alloys, where interstitial sites of metal lattices are occupied
by hydrogen (H) atoms, randomly at high temperatures and in some regular
ways at lower temperatures.
Frequently, metal lattices themselves undergo
structural changes to accommodate a larger number of H atoms interstitially.
Thus, a variety of phases can exist in M–H binary alloys.
(3) Number and size of interstitial sites
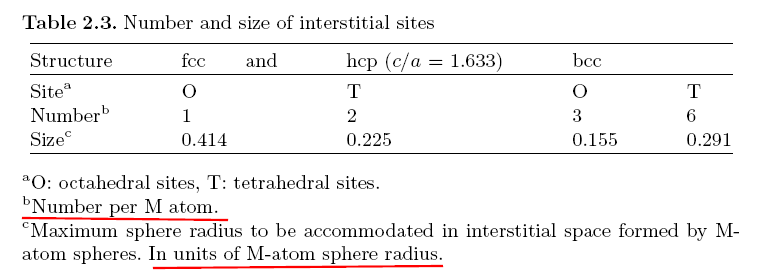

The number of interstitial sites per M atom and the space available for
these sites are given in Table 2.3.
In the fcc lattice, T and O sites are surrounded by regular tetrahedra and
octahedra, respectively, of M atoms.
In the hcp lattice, the polyhedra formed
by the nearest M atoms become distorted as the axial ratio deviates from
the ideal value, c/a = 1.633. 【hcp 结构: M atoms 四面体和八面体是扭曲的】
In the bcc lattice, an O site is surrounded by a
heavily distorted octahedron, having two M atoms at a much closer distance
than the remaining four atoms. Thus, O sites in the bcc lattice are further
divided into OX, OY, OZ sites, depending upon the direction of the four-fold
symmetry axis.
Similarly, T sites in the bcc lattice are also divided into three
groups, TX, TY, TZ, according to their symmetry axis.
(4) the structure of transition metals
For describing the systematics of hydride structures, it is expedient here
to review briefly the structure of transition metals, as listed in Table 2.4 [2.4].
The general systematics is apparent.
Complex allotropic transitions in Mn
and Fe are due to the effect of magnetism.
Among the trivalent lanthanide
metals, Eu and Yb often behave differently from others because their ion
cores prefer to be divalent due to the extreme stability of half-filled and
completely-filled 4f-shell configurations.
These structures occurring under
normal pressure tend to become more compact ones under high pressures.
For Fe, for example, dhcp and hcp structures appear above ∼8GPa (see
Fig. 4.35).
(5) The structure of hydrides depends on the matrix metal, hydrogen concentration,
temperature and pressure.
The structure of hydrides depends on the matrix metal, hydrogen concentration,
temperature and pressure. In cases where the solubility is limited,
certain hydrides could be realized only under high hydrogen pressures. Here
we focus attention solely on the structure and site occupancy of the hydrides
attained so far, without regard to the condition of their synthesis. Individual
high-pressure hydrides are described in Sect. 4.4. The observed structures of
the hydrides are summarized in Table 2.5.
Starting from the right end of the table, fcc and hcp metals (including
Co) absorb hydrogen in O-sites to form a continuous solid solution up to
the monohydride composition x = 1. In some of these cases, substoichiometric
hydrides are formed by the ordering of vacancies (Vacs) in the hydrogen
sublattice. In the Pd-H system, for example, two such ordered structures
[I41/amd and I4/m (H4Vac in Ni4Mo structure)] are known to occur at hydrogen
concentrations higher than the spinodal region, at temperatures below
about 50K “50K anomaly” [2.7–2.9, 2.88–2.90]. The fcc monohydride structure
of O-site occupancy (NaCl type) was also observed in many other cases
(V, Nb, Ta, Cr, Mo, Mn, Fe, Co), always as a high-temperature phase at
high pressures (Sect. 4.4.1). In hcp metals with H atoms on O-sites (NiAs
type), substoichiometric hydrides having anti-CdI2 type structure were observed
in Mn–H,Tc–H and Re–H systems, near the composition [H]/[M] ∼0.5
(Sect. 4.4.1). It may be added that in noble metal Cu, a covalent monohydride
of Wurtzite structure (CuhHt) is formed by chemical synthesis in aqueous solution
[2.3], whereas an fcc hydride, probably represented by CufHo
x, is formed
under high hydrogen pressures (Sect. 4.4.1
In bcc metals, H atoms at low concentrations occupy T sites randomly, but
at high concentrations they tend to occupy certain sublattice(s) of interstitial
sites at low temperatures, causing a small overall distortion of the lattice. In
fact, this lattice distortion removes the degeneracy of the site energy in the
original cubic lattice, allowing the occupancy of a certain interstitial sublattice
among others. For example, in the β phase of the V–H(D) system
(Fig. 2.3), one of the cube axes (Z axis) is elongated by about 10% by occupation
of Oz sites, the energy of which has been lowered in turn by the overall
tetragonal distortion. This particular example will be discussed more fully in
Sect. 5.7.2. Hydrides in which H atoms are ordered on O sites in the bcc lattice
include V2H, V3H2, and VH (Vb
2Ho,Vb
3Ho2
, and VbHo, respectively); those in
which they are ordered on T sites include Nb4H3, NbH, Ta2H, Ta4H3, and
TaH (Nbb4
Ht
3, NbbHt, Tab2
Ht, Tab4
Ht
3, and TabHt, respectively). Hydrides having
these stoichiometric compositions can assume completely ordered structures.
Note that, in spite of a large number of interstitial sites potentially
available in the bcc lattice, the composition of these bcc-based hydrides is
rather limited, namely, x < 1. This is a consequence of mutual repulsion
operating between H atoms. The structure of hydrides of the bcc metals V,
Nb, Ta is described in detail in a review article by Schober and Wenzl [2.10],
and more recently in [2.5].
In the hcp solid solution of Ti [2.91], Zr [2.93] and rare-earth metals (Sc, Y
and lanthanides), H atoms occupy T sites. Unlike the case of O-site occupancy
in Group VI–VIII transition metals, terminal solubilities are rather limited,
and further hydrogenation leads to the formation of dihydrides.
The dihydrides are formed in the CaF2-type sturucture, in which T sites
in the fcc lattice are filled by H atoms (MfHt
2). All the dihydrides listed
here except EuH2 and YbH2 have this structure. These two hydrides are, in
fact, ionic compounds having the same structure as alkaline-earth dihydrides
[2.3, 2.4]. The dihydrides of the CaF2 structure are formed either from bcc
or hcp metals. These dihydrides cannot be reached by successive filling of T
sites of an fcc metal (with the only exception of Cef ).
In Ti, Zr, and Hf, tetragonal distortion of the CaF2 structure, in which one
of the crystallographic axes is shortened, appears at H concentrations higher
than the critical values, x = 1.96, 1.66, and 1.86, respectively, depending
slightly on the temperature. The axial ratio c/a decreases with increasing
H concentration, reaching 0.945, 0.984, and 0.887, respectively, at x = 2.0
[2.1, 2.2].
Regarding the hydrides of rare-earth metals Sc, Y, and lanthanides, compilation
of data by Arons [2.94,2.97] was substantially updated in more recent
review articles of Vajda [2.73] and Vajda and Daou [2.74]. These metals can
be roughly classified into two groups according to the relative stability of
different hydride phases. A schematic view of the phase diagrams of these
systems is shown in Fig. 2.15. There is always a certain range of compositions
where the fcc dihydride phase is stable. In lighter lanthanides, La, Ce
and Pr, the fcc phase field extends to the trihydride composition. For heavier
lanthanides, the fcc phase field becomes narrower; from x ≈ 2.5 for Nd to
x ≈ 2.03 for Lu, and their trihydrides are formed in the hexagonal structure.
A vertical solvus line in some of these cases, a consequence of the ordering of
H atoms, will be discussed in Sects. 2.4.3 and 5.6.1.
The structure of cubic dihydrides can be written as MfHt
2Ho
β. The accommodation
of excess D atoms (x > 2) in O sites was found by very early experiments
of neutron diffraction on CeD2.48 [2.92]. The generality of this result
was questioned by subsequent experiments on various other systems, which
suggested that the occupation of O-sites started in many cases before T-sites
were completely filled. This question of site occupancy was clearly resolved
by more recent experiments of a French group [2.73, 2.74]. Taking sufficient
care for the purity and perfection of samples, and noting that different site
occupancies could be clearly discriminated by different equilibrium pressures,
they showed unambiguously that in ideal situations H atoms occupy T-sites
up to x = 2.0, and additional H atoms enter O-sites. In impure specimens,
the total number of available T sites were less than x = 2.0, because a certain
number of T sites were blocked by impurity atoms.
In many cases, a slight tetragonal distortion takes place as a result of
ordering of H atoms on O sites (Ho). Thus the unit cell is doubled in the c
direction in La, Ce and Sm dihydrides [2.98, 2.101], and in TbD2.25 the Ho
sublattice forms a DO22 (Ni3Mo-type) structure [2.102, 2.104]. A few other
tetragonal phases have been identified between the dihydride and trihydride
phases of Ce [2.105].
The structure of cubic trihydrides, MfHt
2Ho, is sometimes referred to as
BiF3 structure. Note, however, that this strucure cannot be formed in practice
by successive filling of O sites in dihydrides (MfHt
2); a series of tetragonal
phases must be traversed before reaching the trihydride phase. In Ce, the
cubic trihydride phase starts at x = 2.65 at room temperature [2.105].
One peculiar feature of the cubic dihydride phase is that the volume
decreases with excess H. This is believed to be the result of different bonding
character of the H atoms on O sites and T sites (Sect. 7.4).
Hexagonal trihydrides are generally considered as having the structure
MhHt
2Ho. Neutron diffraction experiments on HoD3 have shown, however,
that its structure is slightly different. The positions of H atoms are displaced
slightly from the original T and O sites, making the unit cell three times
larger [2.106]. Other hexagonal trihydrides have also been believed to assume
the HoD3 structure, but more recent work on YD3 suggested that its structure
might be slightly different [2.107, 2.108]. In contrast to cubic trihydrides, the
volume of hexagonal trihydrides is larger by 8 ∼ 10% than the corresponding
dihydrides.
Here we do not deal with hydrides of actinide elements, but briefly describe
their general trend. Actinide elements are usually classified into the
early-actinides (Ac, Th, Pa, U) and the late- (transuranium) actinides (Np,
Pu, Am, Cm, Bk, Cf, etc.). In all the actinides studied so far, up to californium,
fcc dihydrides MH2+β are formed, and in the late-actinides up to
berkelium hcp trihydrides MH3−γ are formed. The site occupancy has not
always been determined, but is believed to be the same as in rare-earth
hydrides. The first two of the early-actinides, actinium and thorium, lacking
5f electrons, behave in many respects as an extension of trivalent Sc, Y
and La, and tetravalent Ti, Zr and Hr, respectively. In protoactinium and
uranium, delocalized 5f electrons having a broad energy band, dip below the
Fermi level, and together with strongly hybridized s-d electrons contribute
significantly to the bonding. The participation of 5f orbitals to the bonding
leads to a diversity of hydride phases and structures in the early-actinides. In
the late-actinides, as more electrons are added, narrowing of the 5f band proceeds
through neptunium and plutonium, and is almost completed in americium.
The 5f electrons are localized and no longer participate in the bonding.
The situation is very similar to lanthanides, where inner-shell 4f electrons do
not participate in the bonding. Thus many properties of the late-actinides
follow the systematics of the lanthanides.
Now, a fairly large body of experimental data has been accumulated on
actinide hydrides. For information on individual systems, the reader is referred
to [2.1, 2.5]. There are many peculiar hydrides formed from actinide
metals, Th4H15 being one of such examples. This hydride, having the highest
H concentration among all the metallic hydrides, once attracted attention
because of its superconducting properties [2.109, 2.110]. An interesting and
useful comparison of actinide and lanthanide hydrides was made in the review
paper by Ward and Haschke [2.111].
https://blog.sciencenet.cn/blog-567091-865744.html
上一篇:温故知新:熵乘以温度项
下一篇:聚变能源研究之托卡马克:第一壁材料/面向等离子材料存在的问题