博文
Nano Res.[生物]│汕头大学张薄博教授/杨琼琼讲师团队:一种盔甲化益生菌靶向递送系统治疗炎症性肠病新路径
||

背景介绍
溃疡性结肠炎(UC)作为炎症性肠病的常见类型,是全球高发的慢性肠道炎症疾病。该病不仅难以根治,长期反复发作还可能进展为结直肠癌,给患者带来沉重的身心负担。目前临床常用的药物治疗方案,普遍存在吸收效果差、潜在毒性强、长期使用副作用显著等问题,无法满足患者的治疗需求。近年来,益生菌疗法因天然安全的特性,在肠道疾病治疗领域展现出巨大潜力,但胃肠道的 “严苛环境” 却成为其发挥作用的 “拦路虎”。口服的益生菌需闯过胃酸、胆盐、消化酶等多重 “关卡”,而溃疡性结肠炎患者肠道内过高的活性氧(ROS)和受损的黏膜层,更会进一步降低益生菌的存活率与定殖能力。尽管现有益生菌包封技术能提升其口服耐受性,但在肠道定殖、生物利用度及治疗效果上仍面临瓶颈。
成果简介
针对益生菌活性差、难定殖等痛点,研究团队创新采用单细胞双层包被技术,为益生菌打造了一副坚固的 “防护盔甲”:首先用二氧化锰纳米酶(一种可高效清除活性氧的纳米材料)对益生菌进行修饰,再包裹上能响应结肠环境的降解材料。这一设计让益生菌具备了“三重本领”—— 既能抵御胃酸、消化酶等极端环境的侵袭,维持自身活性;又能通过纳米酶清除肠道内的有害活性氧,改善病理环境;更能借助结肠响应性材料,在炎症部位通过pH值与活性氧的双重触发实现“精准唤醒”,确保益生菌在病灶处高效发挥作用。实验证实,这种“盔甲益生菌”可有效缓解肠道炎症、修复受损的结肠上皮屏障,同时调节紊乱的肠道微生物群,在溃疡性结肠炎的预防与治疗中均表现出优异效果。值得关注的是,该系统中的纳米酶可与多种益生菌菌株兼容,且能延长益生菌在胃肠道的转运时间,为构建多功能治疗平台奠定了基础。此次研发的“盔甲益生菌”不仅突破了传统益生菌疗法的应用局限,更凸显了其在其他炎症性疾病治疗中的广阔潜力。
图文导读
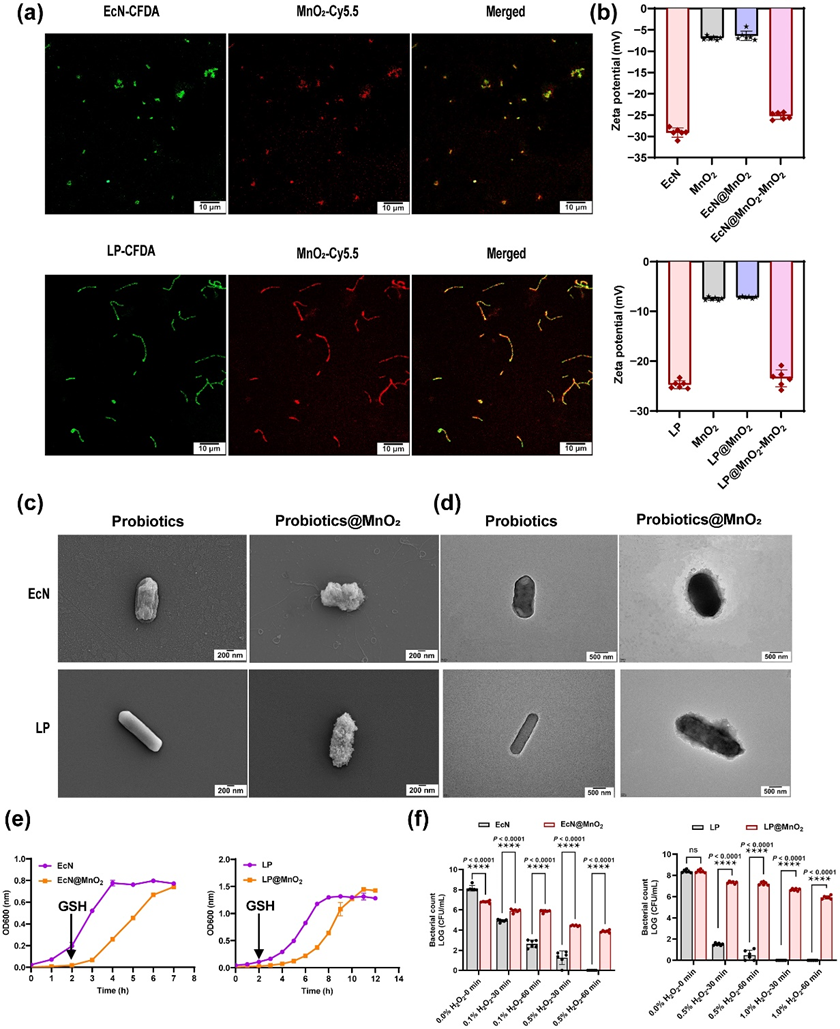
Figure 2 Characterization of probiotics and probiotics@MnO2. (a) Representative CLSM images of probiotics and probiotics@MnO2. The green color represents probiotics cells, which were labeled with 5(6)-carboxyfluorescein diacetate (CFDA). The red color represents MnO2 labeled with bovine serum albumin conjugated with cy5.5. Scale bars: 10 µm. (b) Zeta potential of MnO2, probiotics, probiotics@MnO2, and probiotics@MnO2 -MnO2 measured by DLS (n = 6). (c) Representative SEM images of probiotics and probiotics@MnO2. Scale bars: 200 nm. (d) Representative TEM images of probiotics and probiotics@MnO2. Scale bars: 500 nm. (e) Growth curves of probiotics and probiotics@MnO2 in LB or MRS mediums at 37°C, and the OD600 was monitored using UV spectrometry (n = 3). (f) Cell viability of probiotics and probiotics@MnO2 after incubation with H2O2 for different times (n = 6). Data are presented as the mean ± SD. Statistical analysis was evaluated with two-way ANOVA, giving P values, nsP > 0.05, ****P < 0.0001. CFU, colony-forming units; EcN, E. coli Nissle 1917; LP, L. paracasei; EcN@MnO2, E. coli Nissle 1917 coated with MnO2; LP@MnO2, L. paracasei coated with MnO2; EcN@MnO2 -MnO2, LP@MnO2 -MnO2, remove MnO2 shell via GSH.
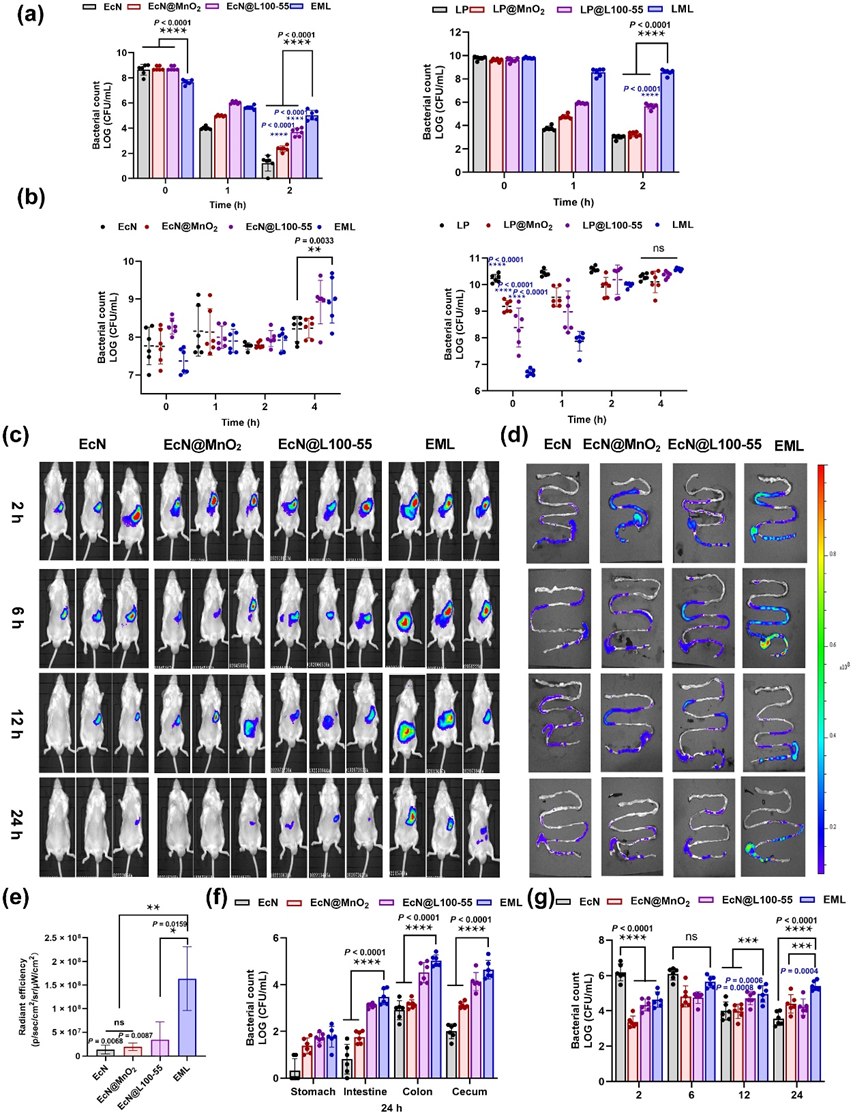
Figure 3 Bioavailability of native probiotics and coated probiotics in vitro and in vivo. (a) Numbers of survived probiotics after exposure to SGF (pH 2 and 3.2 g/L pepsin) at 37°C and 100 rpm for 2 h, and (b) SIF (pH 6.8 and 10 g/L trypsin) at 37°C and 100 rpm for 4 h (n = 6). (c) IVIS bioluminescence images of mice and (d) their GI tracts at different time points after administration of DiR-labeled EcN, EcN@MnO2, EcN@L100-55, and EML. (e) Quantification evaluation of fluorescence signals of EcN, EcN@MnO2, EcN@L100-55, and EML in mice at 24 h (n = 3). (f) Quantification analysis of living EcN in the stomach, intestine, colon, and cecum 24 h post-oral gavage by EcN, EcN@MnO2, EcN@L100-55, and EML (n = 6). (g) Quantification analysis of living EcN in mouse feces at different time points (n = 6). Data are presented as the mean ± SD. Statistical analysis was evaluated with two-way ANOVA for (a), (b), (g), and (f). Statistical analysis was evaluated with Ordinary one-way ANOVA for (e), nsP > 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. The blue asterisk in (a) and (b) indicates the significance between native probiotics and probiotics@MnO2 or probiotics@L100-55. EcN@L100-55, E. coli Nissle 1917 coated with Eudragit L100-55; LP@L100-55, L. paracasei coated with Eudragit L100-55
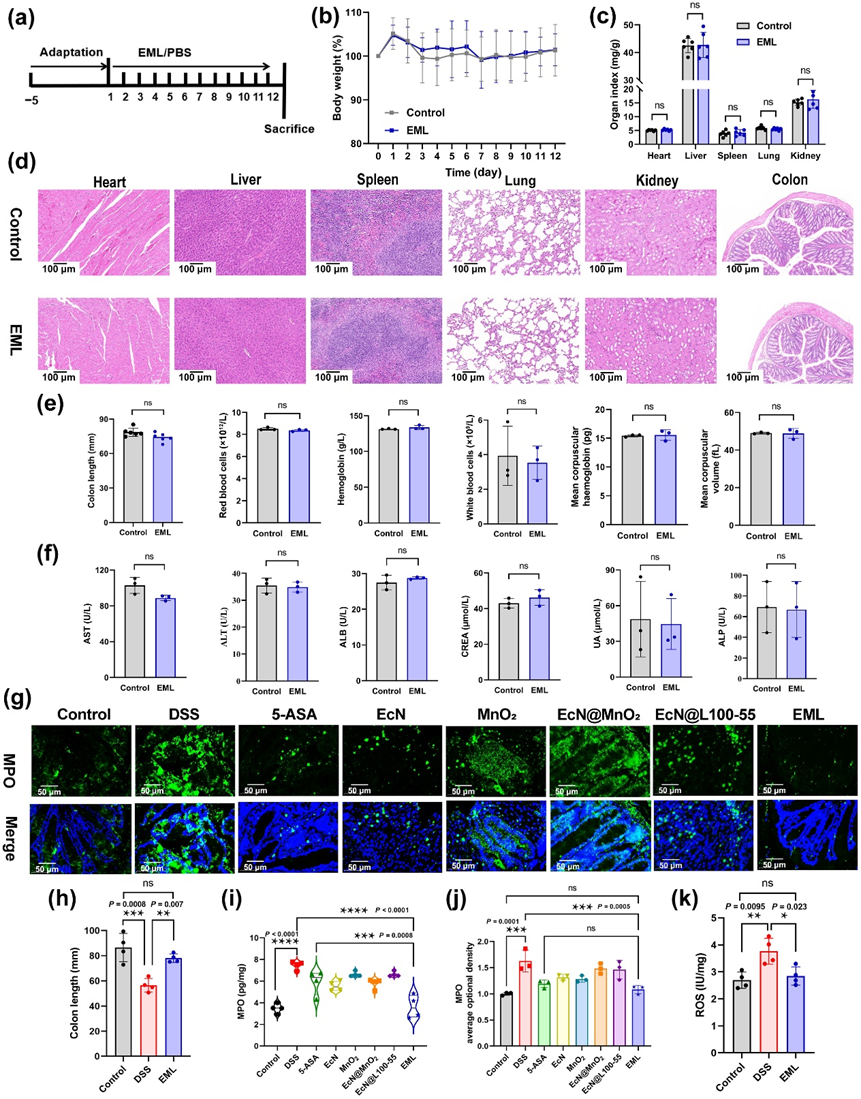
Figure 4 Biosafety evaluation of EML and ROS in vivo evaluation. (a) Schematic showing the experiment procedure for the biosafety evaluation. BALB/c mice were acclimatized for 5 days. Then, the mice were treated with PBS and EML (oral gavage for 12 days; 5 × 107 CFU/mouse/day). (b) The body weight of mice treated with PBS or EML from day 1 to 12. (c) Organ index of control and EML treated mice (n = 6). (d) Representative H&E staining images of heart, liver, spleen, lung, kidney, and colon tissues harvested on day 13 after different treatments from 6 biologically independent animals in each group. Scale bars: 100 μm. (e) Complete blood and (f) serum biochemistry data were obtained on day 13 after EML treatment. Data are presented as mean ± SD (n = 3). Statistical analysis was evaluated with two-tailed Student’s t tests (nsP > 0.05). (g) Colonic MPO protein staining to evaluate intestinal inflammation. Scale bars: 50 μm. (h) Corresponding quantified lengths of colons harvested from mice 12 days after different treatments (n = 4). (i) The level of MPO in the colon tissues measured by ELISA (n = 4). (j) Semiquantitative analysis of the expression of MPO in the colon based on immunofluorescence staining (n = 3). (k) The levels of ROS in the colon tissues measured by ELISA (n = 4). Data are presented as mean ± SD. Statistical analysis was evaluated with Ordinary one-way ANOVA (nsP > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001) for (c), (h)–(k).
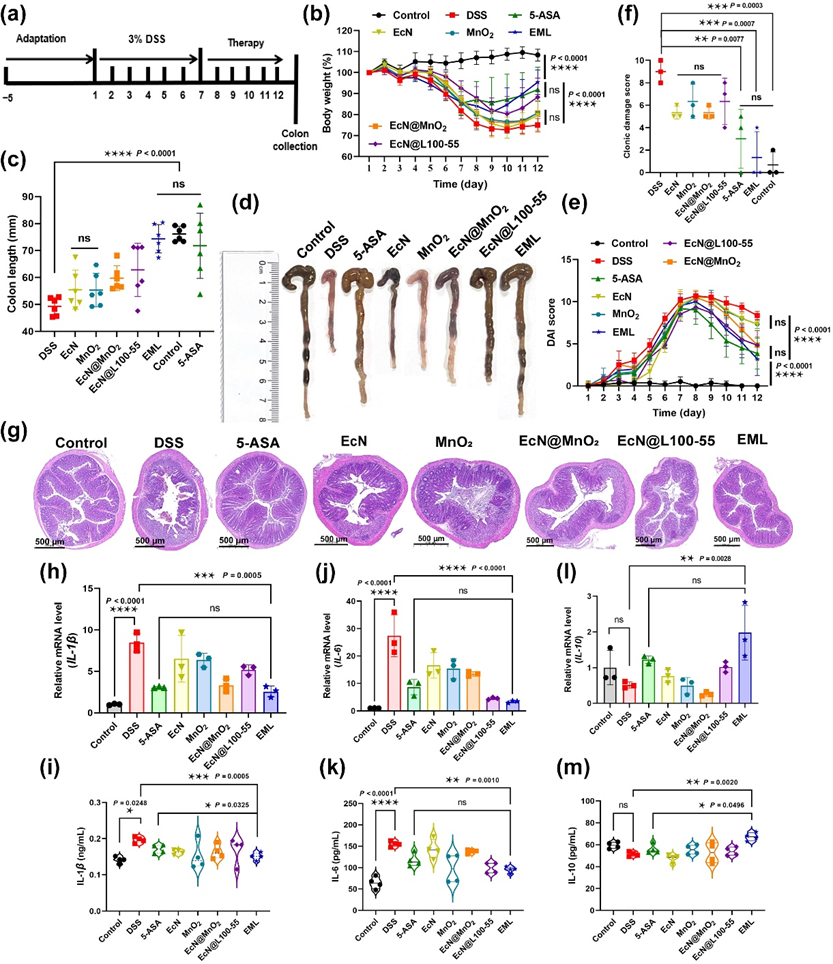
Figure 5 Treatment efficacy of EML against DSS-indue murine IBDs. (a) Schematic showing the experiment procedure for the treatment of DSS-induced IBD mice. BALB/c mice were acclimatized for 5 days. Then, the mice were fed 3% DSS for 7 days. Thereafter, the mice were treated with PBS, 5-ASA (5 mg/mouse/day), EcN, MnO2, EcN@MnO2, EcN@L100-55, or EML (oral gavage for 5 days; 5 × 107 CFU/mouse/day). (b) The body weight of mice during the treatment (n = 6). (c) Corresponding quantified lengths (n = 6) and (d) photographs of colons harvested from mice after different treatments on day 13. (e) The DAI scores of mice after the treatment (n = 6). (f) The colonic damage scores of mice after different treatments (n = 3). (g) Representative H&E staining images of colon tissues harvested on day 13 after different treatments from 6 biologically independent animals in each group. Scale bars: 500 μm. The levels of (h) IL-1β, (j) IL-6, and (l) IL-10 in the colon tissues measured by RT-qPCR on day 13 (n = 3). The levels of (i) IL-1β, (k) IL-6, and (m) IL-10 in the serum measured by ELISA on day 13 (n = 4). Data are presented as the mean ± SD. Statistical analysis was evaluated with two-way ANOVA for (b) and (e). Statistical analysis was evaluated with Ordinary one-way ANOVA for (c), (f), and (h)–(m). nsP > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
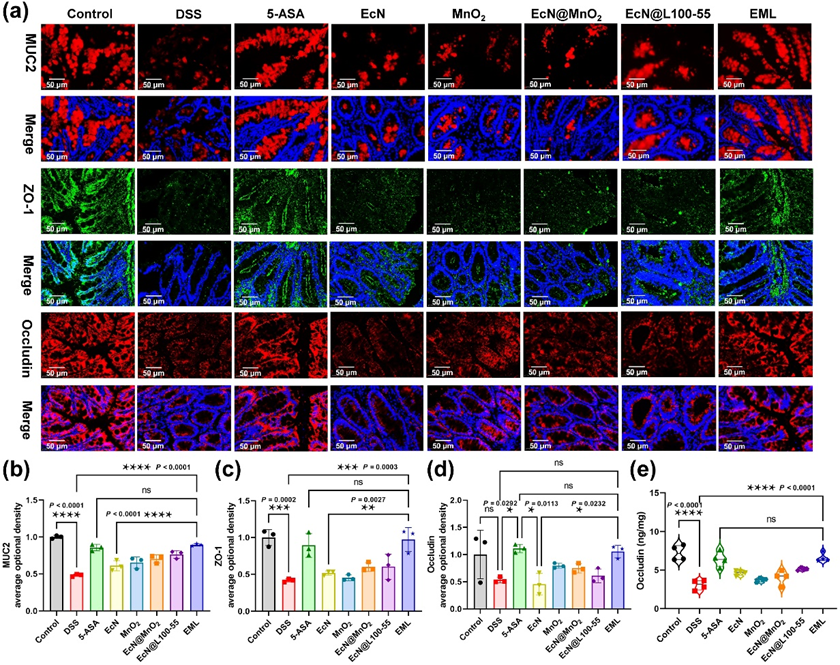
Figure 6 Repair effect of EML against DSS-induced intestinal barrier damage. (a) Colonic mucin glycoprotein and tight-junction proteins staining to evaluate intestinal barrier damage. Scale bar: 50 μm. (B, C and D) Semiquantitative analysis of the expression of MUC2 (b), ZO-1 (Cc), and Occludin (d) in the colon based on immunofluorescence staining. (e) The levels of Occludin in the colon tissues measured by ELISA. Data are presented as the mean ± SD (n = 3). Statistical analysis was evaluated with Ordinary one-way ANOVA (nsP > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001) for B-E.
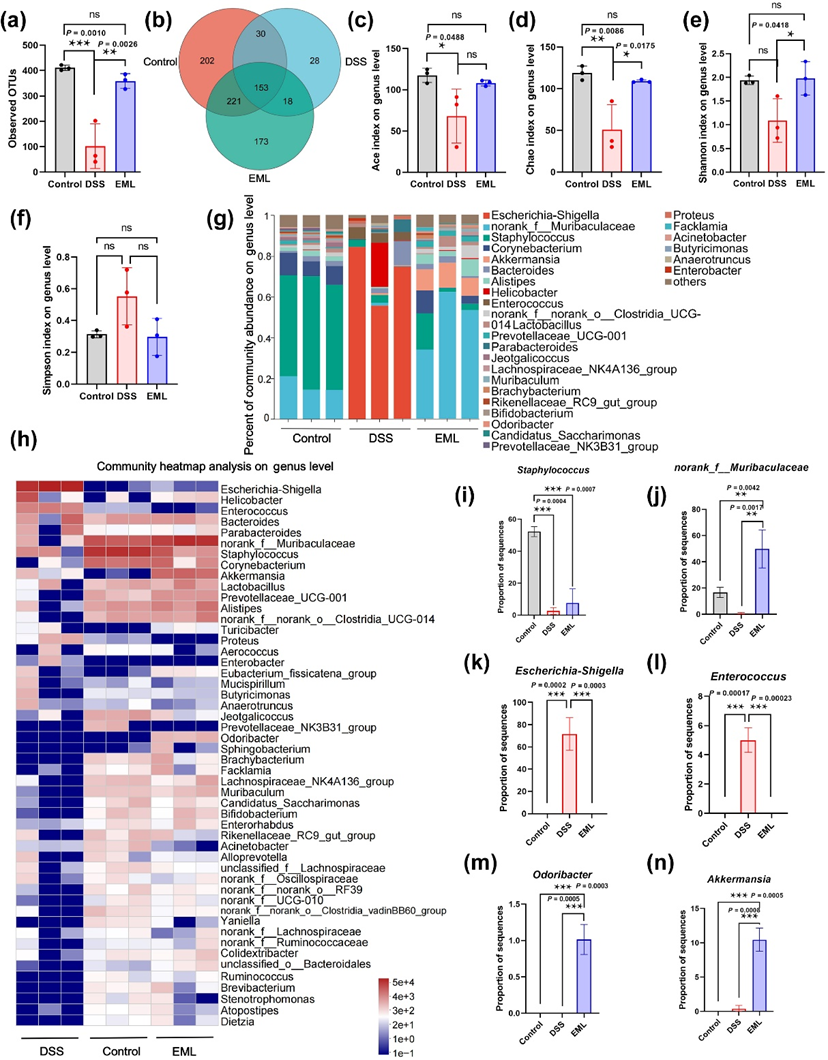
Figure 7 Modulation of gut bacteria by EML during colitis treatment. (a, b, c, d, e and f) Comparison of alpha diversity assessed by (a) observed OTUs, (b) Venn diagram, (c) Ace index, (d) Chao 1 index, (e) Shannon index, and (f) Simpson index. (g) Column diagram of the relative abundance of gut microbiome at the genus level. (h) Heatmap illustration of gut microbial distribution at the genus level. (i, j, k, l, m and n) Relative abundance of Staphylococcus (i) norank_f_Muribaculaceae (j), E.-Shigella (k), Enterococcus (l), Odoribacter (m), and Akkermansia (n). Data are presented as the mean ± SD (n = 3). Statistical analysis was evaluated with Ordinary one-way ANOVA (nsP > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001) for A-F, and I-N.
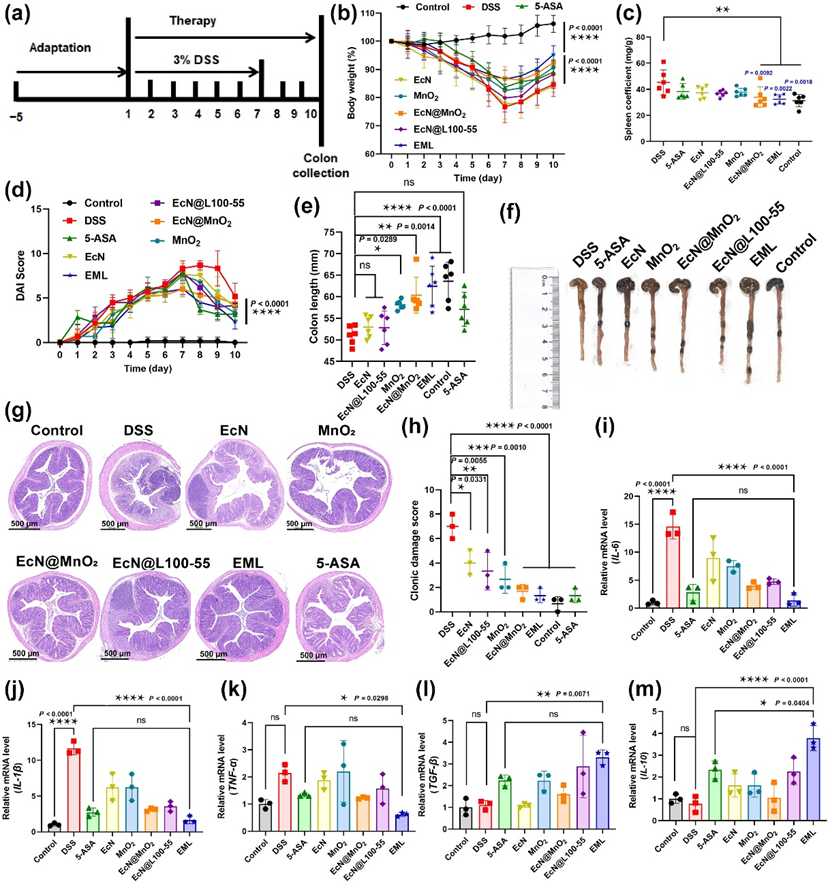
Figure 8 Preventative efficacy of EML against DSS-indue murine IBDs. (a) Schematic showing the experiment procedure for the treatment of DSS-induced IBD mice. BALB/c mice were acclimatized for 5 days. Then, 3% DSS was administered continuously via drinking water for 7 days. Meanwhile, the mice were treated with PBS, 5-ASA (5 mg/mouse/day), EcN, MnO2, EcN@MnO2, EcN@L100-55, or EML (oral gavage for 10 days; 5×107 CFU/mouse/day). (b) The body weight of mice during the treatment. (c) The spleen coefficient of mice after different treatments. (d) The DAI scores of mice after the treatment. (E and F) Quantified lengths of colons (e) and corresponding photographs (f) harvested from mice after different treatments on day 11. (g) Representative H&E staining images of colon tissues harvested on day 11 after different treatments from 6 biologically independent animals in each group. (h) The colonic damage score of mice after different treatments. (i-m) The levels of IL-6, IL-1β, TNF-α, TGF-β, and IL-10 in the colon tissues measured by RT-qPCR on day 11. Data are presented as the mean ± SD (n = 6 biologically independent samples for b, c and e, n = 3 biologically independent samples for h-m). Statistical analysis was evaluated with two-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001) for b, d, i, j, k, l, and m. Statistical analysis was evaluated with Ordinary one-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001) for c, e, and h. DSS, dextran sodium sulfate; 5-ASA, 5-aminosalicylic acids; EcN, E. coli Nissle 1917; EcN@MnO2, E. coli Nissle 1917 coated with MnO2; EcN@L100-55, E. coli Nissle 1917 coated with Eudragit L100-55; EML, E. coli Nissle 1917 coated with MnO2 and Eudragit L100-55.
作者简介
张薄博,教授,博士生导师。1982年10月,男,广东汕头人,华南理工大学本硕,香港中文大学博士,欧洲科学院苏宝连院士课题组博士后。2012-2019年就职于江南大学生物工程学院,2020年1月起就职于汕头大学生物系。
主要从事微生物发酵,食品科学与生物医药方向的研究。已主持国家重点研发计划子课题、国家自然科学基金面上项目等纵向科研项目10项,企业横向课题12项。已在ACS Nano,Natural Product Reports,Chemical Engineering Journal,Journal of Agricultural and Food Chemistry等相关学科权威期刊上以第一/通讯作者发表SCI论文50余篇(其中包括自然指数期刊Nature Index论文3篇,中国科学院一区论文18篇,ESI高被引论文2篇),作为参与人获得福建省科技进步二等奖1项,申请国家发明专利16项,其中 8 项已获授权,有4项研究成果已通过企业合作转化为实际生产应用。
入选江苏省“六大人才高峰”高层次人才、“双创计划”科技副总,汕头市高层次人才,兼任广东省食品学会理事,广东省生物化学与分子生物学学会理事,汕头博士会药品与健康食品专业委员会常务副主任,汕头大学学术委员会委员,中国科学院二区SCI期刊Frontiers in Microbiology的副编辑等职务。 联系方式:Email: bbzhang@stu.edu.cn 电话/微信:15206191336
杨琼琼,博士,现任汕头大学理学院讲师。主要从事食品科学与营养领域的研究工作,聚焦于功能性食品成分的稳态化与靶向递送、纳米载药系统设计及在炎症性肠病治疗中的应用等方向。
在科研工作中,杨琼琼作为核心研究人员,系统开展了姜黄素等天然活性成分的包埋、递送与生物效应评价研究,主持国家自然科学基金青年基金和省部级纵向课题3项,横向课题2项,研发了多种具有ROS响应性的智能递送系统,显著提高了活性成分的稳定性、生物可及性与靶向性。以第一发明人申请国家发明专利9项,获授权中国发明专利3项,其中2项相关技术已成功转让。
在基础研究方面,以第一作者或通讯作者在ACS Nano、Chemical Communications、Materials Today Bio、Nano Research、Food Chemistry等国际权威期刊发表SCI论文20篇(其中包括自然指数期刊Nature Index论文2篇,中国科学院一区论文13篇),研究成果聚焦食品胶体递送系统构建、机制解析及其抗氧化应激研究,体现了较高的学术水平和创新性。
曾多次获得重要奖项,包括第十届和第八届全国大学生生命科学竞赛优秀指导老师奖(国家级一等奖和二等奖)、博士及硕士研究生国家奖学金,并被评为广西壮族自治区优秀大学毕业生。入选汕头市高层次人才,兼任中国食品学会会员、广东省食品学会会员、美国化学会会员、汕头博士会药品与健康食品专业委员会常务委员等职务。长期致力于推动食品科学与营养领域的科研创新与人才培养工作。联系方式:Email: qiongqiongyang@stu.edu.cn 电话/微信:15221271673
文章信息
Shi J, Zhang Y, Wei X, et al. Sequential responsive single-cell encapsulated probiotics for targeted therapy of inflammatory bowel diseases. Nano Research, 2025, 18(11): 94908003. https://doi.org/10.26599/NR.2025.94908003.

识别二维码或点击左下角“阅读原文”可访问全文

https://blog.sciencenet.cn/blog-3563286-1512502.html
上一篇:Nano Res.[单元]│暨南大学李宝军团队杨先光课题组开发新型纳米纤维传感器:高灵敏度银离子检测与纳米波导技术取得进
下一篇:Nano Res.[器件]│郑州大学毛彦超:基于驻极体纳米纤维摩擦电传感器的非接触VR人机界面