博文
[转载]hLife 2025年第十一期正式出版
||

封面解读
张玉霞
Neonatal liver failure caused by viral infections remains a critical yet understudied challenge in pediatric medicine, with rotavirus infections posing particular risks to newborns. Our study employing rhesus rotavirus (RRV)-infected neonatal mice uncovered disrupted neutrophil development through single-cell RNA sequencing, identifying phosphodiesterase Pde4b as a key upregulated factor. Therapeutic intervention with dipyridamole, a phosphodiesterase (PDE) inhibitor, demonstrated multifaceted benefits: it restored neutrophil differentiation by suppressing excessive activation, promoting apoptosis, and enhancing cellular clearance. Additionally, dipyridamole mitigated harmful immune responses by reducing autoreactive B cell activity and cytotoxic lymphocyte activation, collectively improving liver histopathology and survival rates in infected neonates. These findings highlight PDE inhibition as a promising therapeutic avenue for rotavirus-associated diseases, offering potential clinical applications to address this life-threatening condition. The cover story showcases how PDE-targeted therapy comprehensively restores the neonatal liver’s immune microenvironment, revitalizing its biological vitality.
导读
All Papers
hLife | 巴西探索新模式突破高价药困局,大幅降低癌症与罕见病治疗成本
通讯作者:Antonio Carlos Campos de Carvalho

巴西正在通过创新、高效且经济的生产与分发模式,在提升先进治疗用药(ATMPs)可及性方面取得重大进展。这些举措由隶属于巴西联邦政府的奥斯瓦尔多·克鲁兹基金会(Fiocruz)主导,旨在大幅降低癌症和罕见遗传病等疾病治疗的高昂费用,减轻医疗系统和政府财政的压力。
引用:
Campos de Carvalho AC, Sachetti CG, Krieger MA. Enabling manufacture and access to advanced therapy medicinal products in low- and middle-income countries. hLife 2025; 3: 521–523.
2. The terra incognita in microbiology for artificial intelligence and where to go next
hLife | 王军研究团队评述AI将点亮微生物研究的未知领域
通讯作者:王军

在人类探索自然的伟大征程中,微生物仍是认知最薄弱、最神秘的领域之一。自17世纪列文虎克首次通过显微镜发现微生物以来,尽管科技不断进步,我们却仍像是举着火炬在黑暗洞穴中摸索——目前对微生物世界的认知尚不足1%。而人工智能(AI)正被寄予厚望,或将成为打开这片“未知地图”的关键钥匙。本文系统梳理了微生物领域面临的三大核心挑战,并提出了AI推动该领域突破瓶颈的未来路径。

引用:
Guo S, Wang J. The terra incognita in microbiology for artificial intelligence and where to go next. hLife 2025; 3: 524–526.
3. From spectrum to species: A new era of medical microbiology with AI-powered Raman spectroscopy
hLife | AI赋能拉曼光谱技术推进医学微生物学研究2.0时代
通讯作者:付钰

本文系统阐述了AI赋能下拉曼光谱技术在病原体识别与表型检测中的创新突破和临床前景,提出了该技术未来还需要进一步发展的方向。
引用:
Yang J, Wang M, Chen Z, et al. Selection and engineering of broad-spectrum antiviral affibody peptides against SARS-CoV-2 variants. hLife 2025; 3: 448–451.
4. Forging trust in AI-assisted disease diagnosis
通讯作者:董迪

本文从责任划分、数据伦理、可解释性等方面梳理了AI在医疗诊断应用中面临的挑战,并探讨了AI通往未来“人机共生”医疗生态的路线。
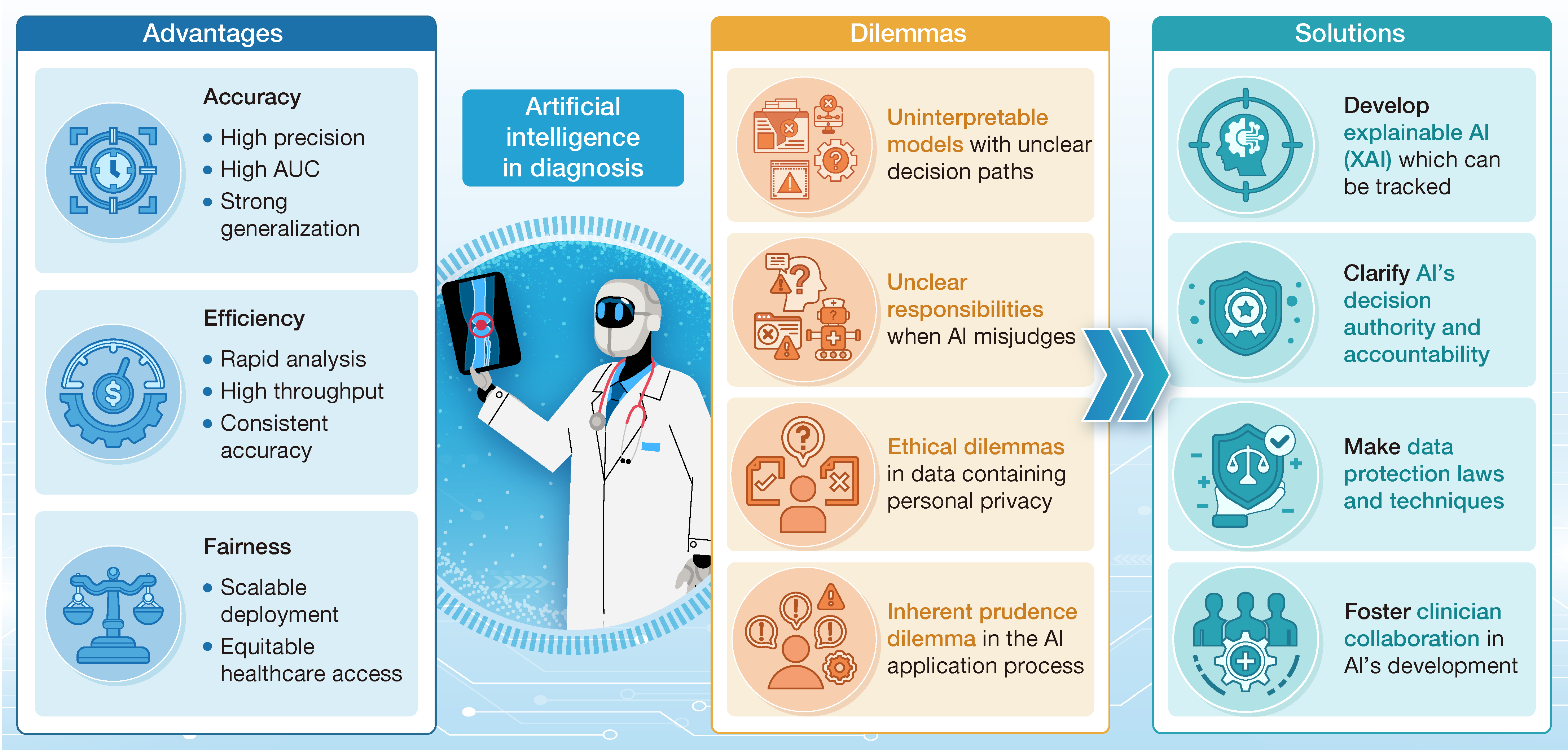
引用:
Zhang Y, Fang M, Feng X, et al. Forging trust in AI-assisted disease diagnosis. hLife 2025; 3: 531–533.
5. Artificial intelligence serves as a research aide in biology but never eclipses humanity as a successor
hLife | 协同进化:AI会取代人类在生物创新领域的主导地位吗?
通讯作者:孙宇、程从超

本文按工作模式类比,将现有AI分为“工具”、“镜子”与“助手”这三种角色来展开分析,通过探索不同模式的边界,讨论了未来“人机协作”中潜在的新挑战和新机会。
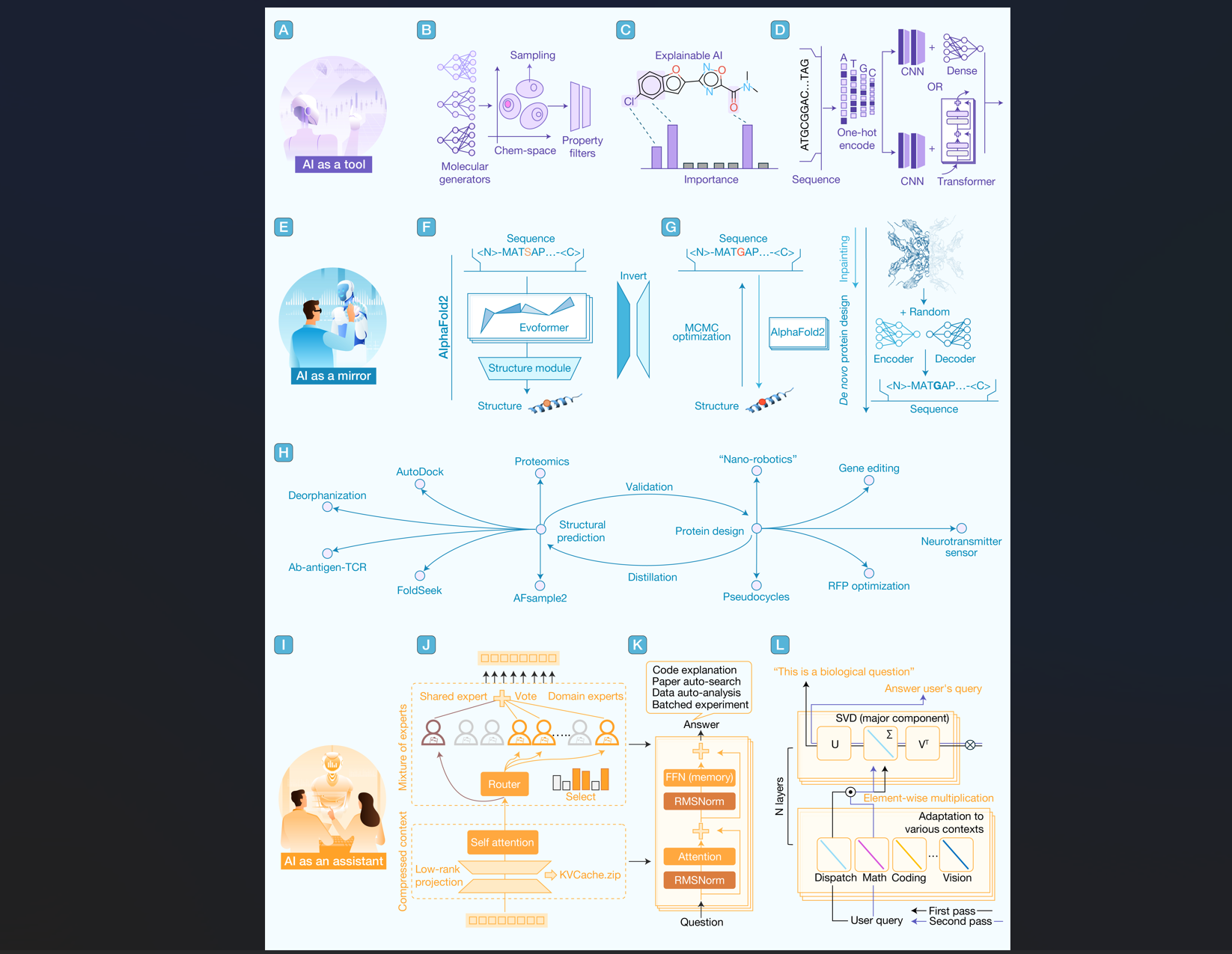
引用:
Yang W, Sun Q, Zhang Y, et al. Artificial intelligence serves as a research aide in biology but never eclipses humanity as a successor. hLife 2025; 3: 534–537.
6. Single-cell transcriptome analysis reveals mechanism of phosphodiesterase inhibitor in neonatal mouse infection-associated liver injury treatment
hLife | 广妇儿张玉霞/温哲研究团队揭示靶向PDE重塑中性粒细胞发育轨迹可治疗新生儿轮状病毒肝损伤
通讯作者:张玉霞,温哲
本文揭示靶向磷酸二酯酶类(Phosphodiesterases,PDEs)抑制剂可调节整体免疫微环境,维持免疫稳态,从而抑制感染引发的异常免疫反应,显著改善新生儿轮状病毒肝损伤,这一成果为临床治疗提供了新靶点。
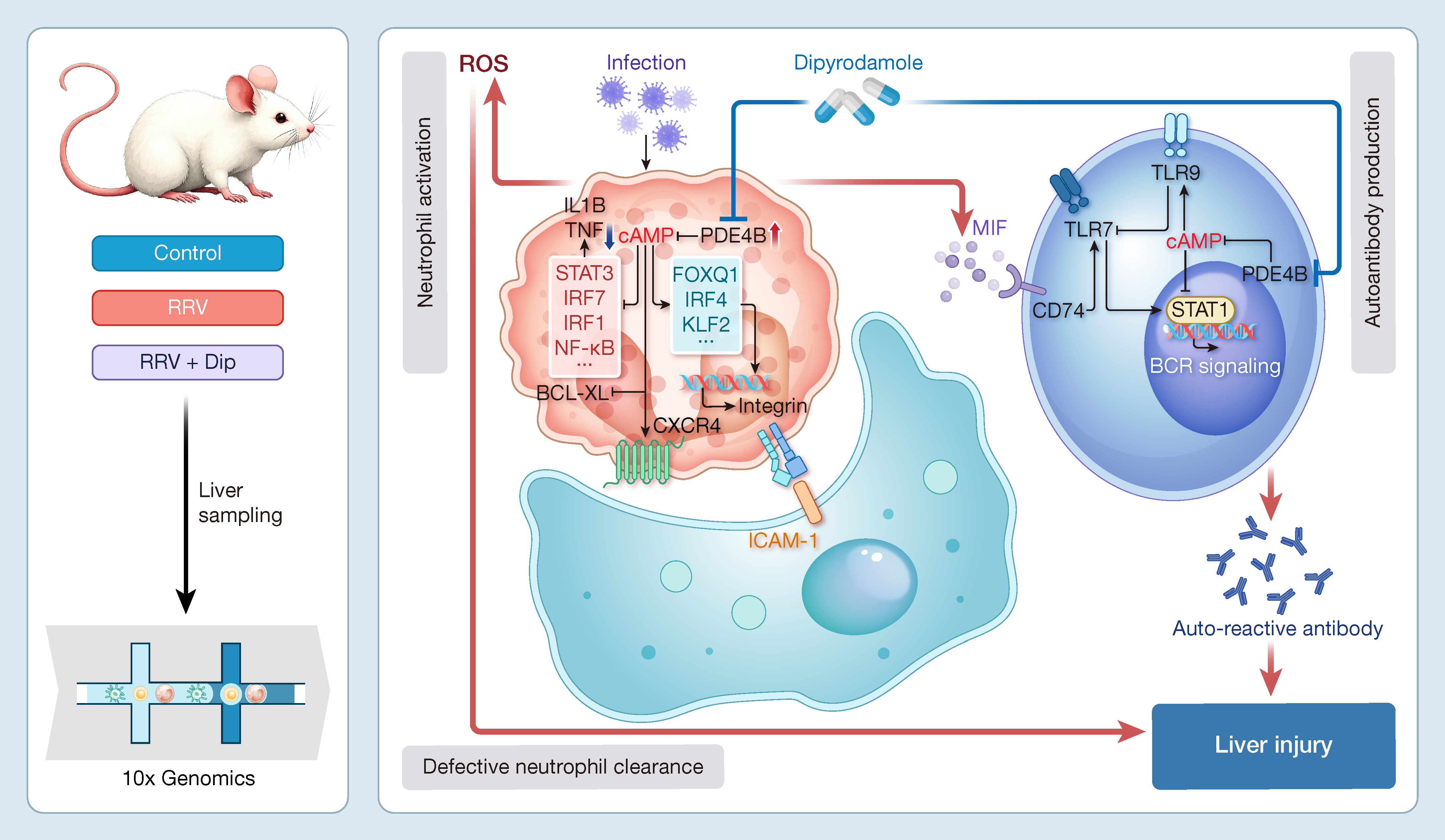
引用:
Chen X, Xu Y, Ren H, et al. Single-cell transcriptome analysis reveals mechanism of phosphodiesterase inhibitor in neonatal mouse infection-associated liver injury treatment. hLife 2025; 3: 538–550.
7. Prophylactic and therapeutic itaconate treatment alleviates COVID-19-associated lung injury
通讯作者:林树海、张天英、夏宁邵

Itaconate, an immunomodulatory metabolite with known anti-inflammatory properties, has underexplored therapeutic or prophylactic potential against coronavirus disease 2019. Using an interanimal transmission golden hamster model of severe acute respiratory syndrome coronavirus 2-induced acute lung injury, we first assessed ITA changes in dNS1-RBD-vaccinated hamsters via metabolomic profiling. Then, we evaluated prophylactic intranasal and therapeutic intraperitoneal ITA administration, assessed by histopathology, transcriptomic, and metabolomic profiling, followed by multi-omics integration, including gene expression clustering, pathway enrichment, and cytokine/chemokine–metabolites correlation analyses. Public bronchoalveolar lavage fluid single-cell RNA-sequencing datasets from COVID-19 patients were re-analyzed to explore macrophage heterogeneity. Intranasal dNS1-RBD vaccine upregulated ITA levels, prompting further exploration of its immunomodulatory role. Both prophylactic and therapeutic ITA treatments significantly mitigated weight loss and improved lung pathology. Correlation analyses implied a potential regulatory crosstalk between fatty acid β-oxidation and reduced inflammatory response. Re-analysis of BALF scRNA-seq dataset highlighted transcriptional networks involving PPARG, RARA, BHLHE41, TCF7L2, and ESRRA—genes linked to macrophage self-renewal and metabolic homeostasis, which appeared to be preserved in ITA-treated hamsters. These findings underscore ITA’s role in modulating immunometabolic responses, particularly through FAO-driven macrophage reprogramming, to attenuate SARS-CoV-2-induced lung damage. Together, this study provides insights into host-directed therapies targeting metabolic reprogramming to mitigate COVID-19 severity.
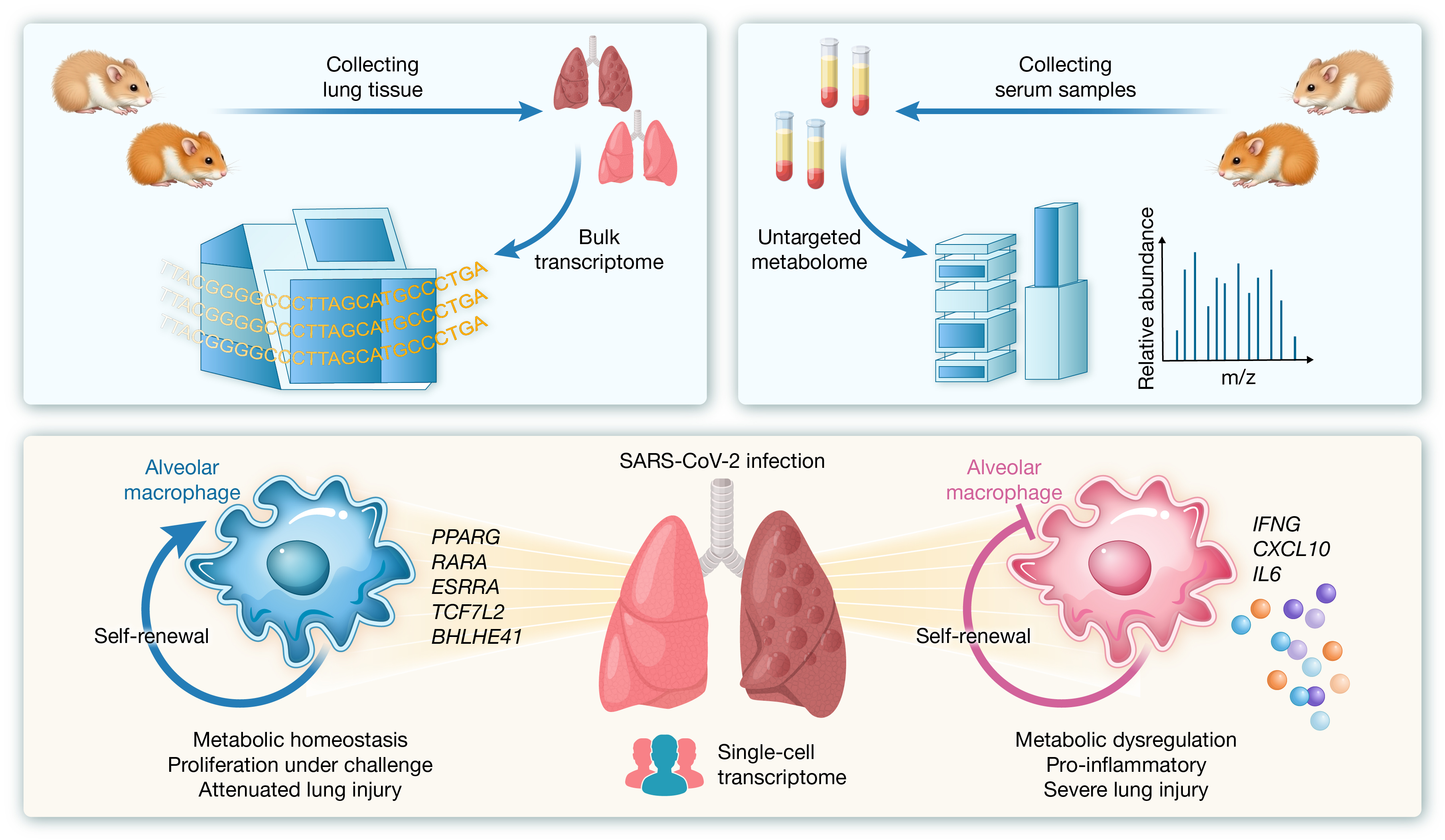
引用:
Jiang Y, Yang P, Yao B, et al. Prophylactic and therapeutic itaconate treatment alleviates COVID-19-associated lung injury. hLife 2025; 3: 551–564.
8. Rational design of human CD26 receptor for a strong neutralizing ability against MjHKU4r-CoV-1 and MERS-CoV
通讯作者:白崇智、苏超、韩鹏程
A novel pangolin-origin Middle East respiratory syndrome coronavirus-like (MERS-like coronavirus), Manis javanica HKU4-related coronavirus 1 (MjHKU4r-CoV-1), has recently been identified. This virus utilizes dipeptidyl peptidase 4 (DPP4 or CD26) as its entry receptor and exhibits high infectivity in human cells and organs. However, no therapeutic agents have been developed to inhibit a potential outbreak of MjHKU4r-CoV-1. In this study, we determined the Cryo-electron microscopy (Cryo-EM) structure of the MjHKU4r-CoV-1 receptor-binding domain (RBD) in complex with human CD26 (hCD26), revealing the detailed interactions at the virus-receptor interface. Based on these structural insights, we designed an hCD26 mutant (hCD26mu) with enhanced binding affinity for the MjHKU4r-CoV-1 RBD and demonstrated its potent neutralizing activity against MjHKU4r-CoV-1 pseudovirus. When fused to ferritin as a nanoparticle, the hCD26mu exhibited increased neutralization potency against both MjHKU4r-CoV-1 and MERS-CoV. These findings highlight the potential of the optimized hCD26mu as a therapeutic candidate for preventing MjHKU4r-CoV-1 infection.
引用:
Zhang Y, Tian M, Han Y, et al. Rational design of human CD26 receptor for a strong neutralizing ability against MjHKU4r-CoV-1 and MERS-CoV. hLife 2025; 3: 565–568.
https://blog.sciencenet.cn/blog-3552961-1513872.html
上一篇:[转载]2025年度hLife最美封面评选
下一篇:[转载]hLife | 中科大刘欢团队追溯HIV/AIDS防治科学技术史