博文
[转载]hLife 2025年第九期正式出版
||

封面解读
秦成峰/周超
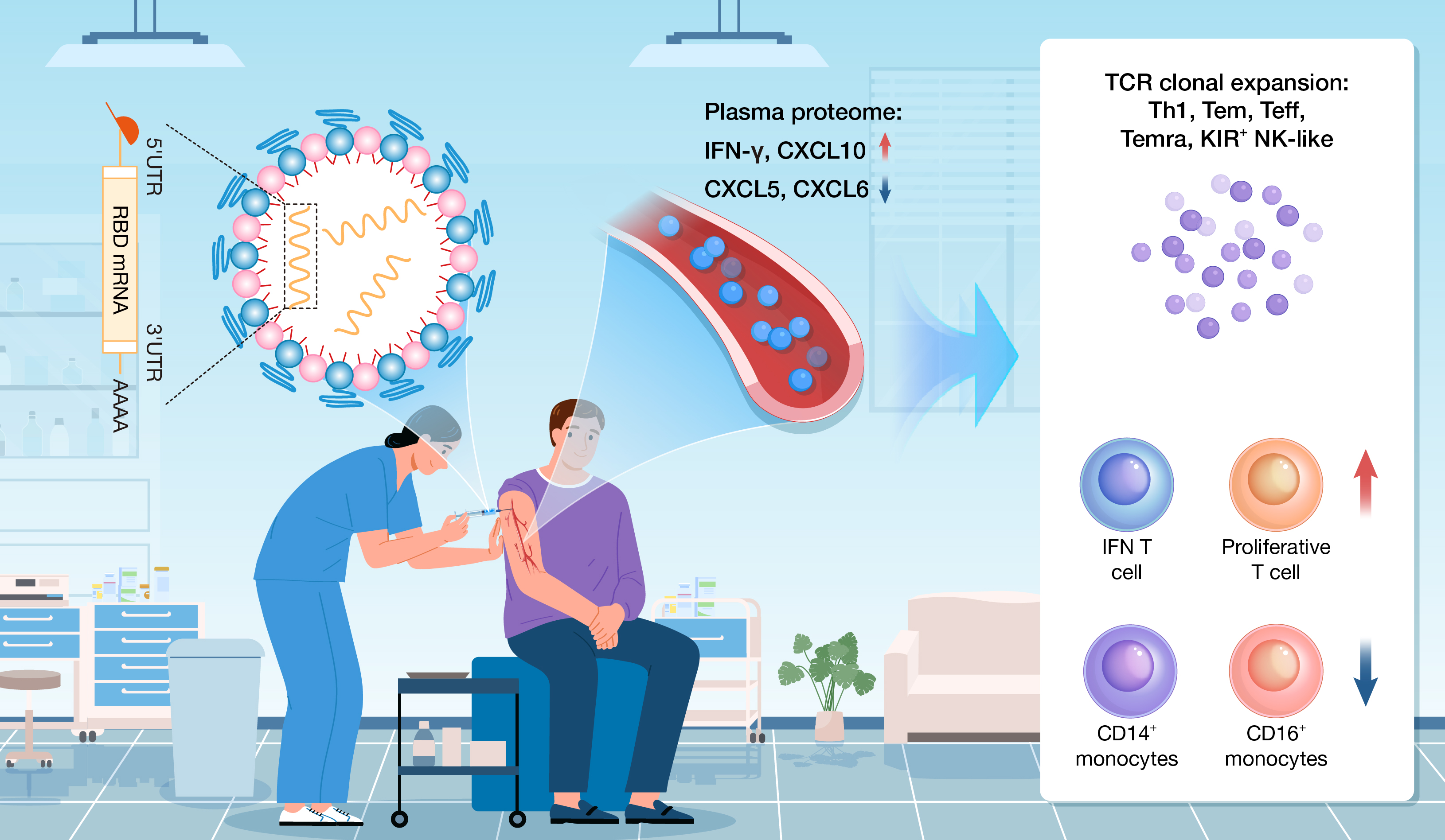
The COVID-19 pandemic, caused by SARS-CoV-2, has led to profound global consequences. The invention of the mRNA vaccine represents a pivotal milestone in both human history and vaccine development. For the first time, these vaccines have been deployed on a large scale, revolutionizing the way we combat infectious diseases. This study focused on ARCoV, a non-modified, Chinese-made mRNA vaccine targeting the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein. ARCoV immunization induced an increased relative abundance of interferon-activated T cells, proliferative T cells, and naïve T cells, along with clonal expansion of effector T cells and killer cell immunoglobulin-like receptor (KIR)-expressing natural killer (NK)-like cells. Monocytes and dendritic cells exhibited activation of the innate immune response. The cover image portrays a winding blood vessel as the “river of time,” where mRNA vaccine particles—depicted as life-saving pods—ignite the activation of diverse immune cells, symbolizing the mRNA vaccine’s dual impact: its transformative role in safeguarding global health and its profound modulation of immune responses within the bloodstream.
All Papers
通讯作者:赵慧、秦成峰

Highlights
•ARCoV vaccination elicits increased expression of C-X-C motif chemokine ligand 10 (CXCL10) and interferon-gamma (IFN-γ).
•Single-cell sequencing shows expansion of interferon-activated and proliferating T cell subsets after immunization.
•ARCoV induces expansion of T cell receptor (TCR) clones in effector T cells and KIR+ natural killer (NK)-like cells.
引用:
Zhou C, Sun M, Huang X, et al. Systemic profiling of immune responses in healthy adults vaccinated with an RBD-targeting COVID-19 mRNA vaccine. hLife 2025; 3: 415–432.
hLife |广州医科大学赵金存/王延群研究团队证实黏膜疫苗抵抗JN.1效力不足六个月
通讯作者:王延群、田新贵、朱爱如、张璐、赵金存
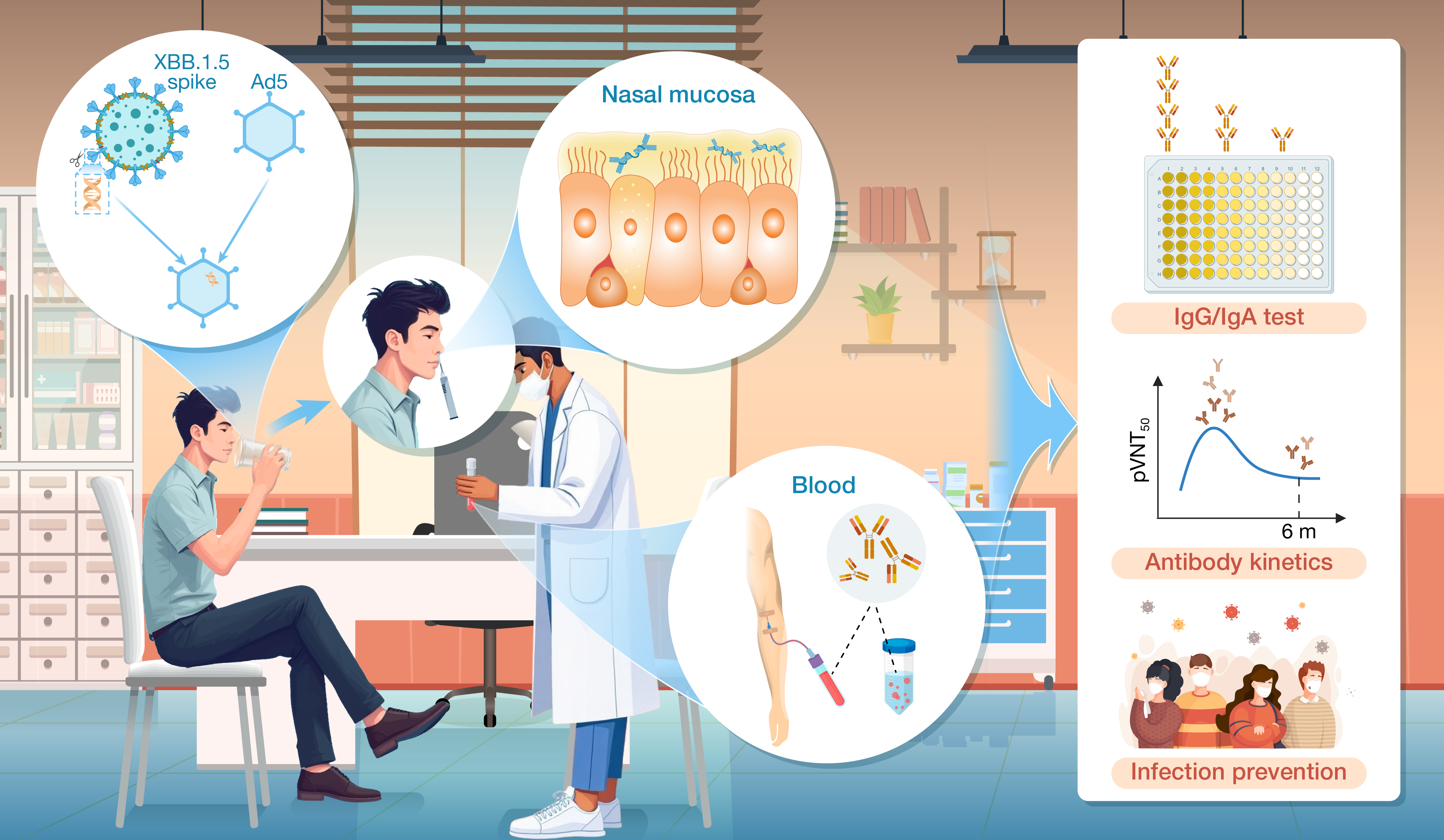
本文评估了单次Ad5-XBB.1.5 黏膜疫苗接种后六个月内的鼻腔和血浆抗体反应,实际跟踪及实验检测发现单次Ad5-XBB.1.5黏膜疫苗能触发黏膜和血浆产生IgA抗体,但疫苗抵抗JN.1感染保护期低于6个月。本研究确定了鼻腔抗体的存在与诱导特性,同时明确了黏膜免疫的保护持续时长,对于理解鼻黏膜部位病毒中和能力及预防传播具有重要意义。
引用:
Wang Y, Wei P, Zhang J, et al. Mucosal adenovirus vaccine Ad5-XBB.1.5 boosting elicits nasal IgA and transiently prevents JN.1 wave infection for less than 6 months in real-world settings. hLife 2025; 3: 433–447.
3. Selection and engineering of broad-spectrum antiviral affibody peptides against SARS-CoV-2 variants
通讯作者:Christopher John Hipolito、齐建勋、施一
In summary, we successfully identified ZSpike:2, a broad-spectrum antiviral affibody targeting SARS-CoV-2 S protein, through mRNA display technology. To improve its neutralizing potency, ZSpike:2 was structurally optimized using three multimerization engineering strategies: a (GGGGS)3-linked homodimer, (ZSpike:2)2 fused with the Fc region of IgG1, and a self-assembled ferritin nanoparticle displaying the (ZSpike:2)2-Fc. The nanoparticle-conjugated construct, (ZSpike:2)2-Fc-ferritin, exhibited broad-spectrum neutralizing activity against all tested SARS-CoV-2 variants, indicating that it has acquired novel neutralization mechanism compared with the (ZSpike:2)2-Fc. We propose that this modification allows (ZSpike:2)2-Fc to assemble into multimeric structures, enhancing its binding avidity to the S protein. Additionally, when (ZSpike:2)2-Fc-ferritin specifically binds to the S protein, the increasing size of (ZSpike:2)2-Fc-ferritin could result in steric hindrance and further block interactions between S protein and ACE2. Moreover, the increasing size of (ZSpike:2)2-Fc-ferritin likely contributes to its broad-spectrum activity by conferring an alternative functional mechanism. This modification strategy is not only applicable to ZSpike:2 but can also be extended to other antiviral protein-binder agents, such as monobodies and nanobodies. Furthermore, the approach of conjugating different antiviral agents with nanoparticles holds promise for developing novel broad-spectrum antiviral drugs. Future studies will assess the in vivo efficacy of (ZSpike:2)2-Fc-ferritin and explore its underlying antiviral mechanisms.
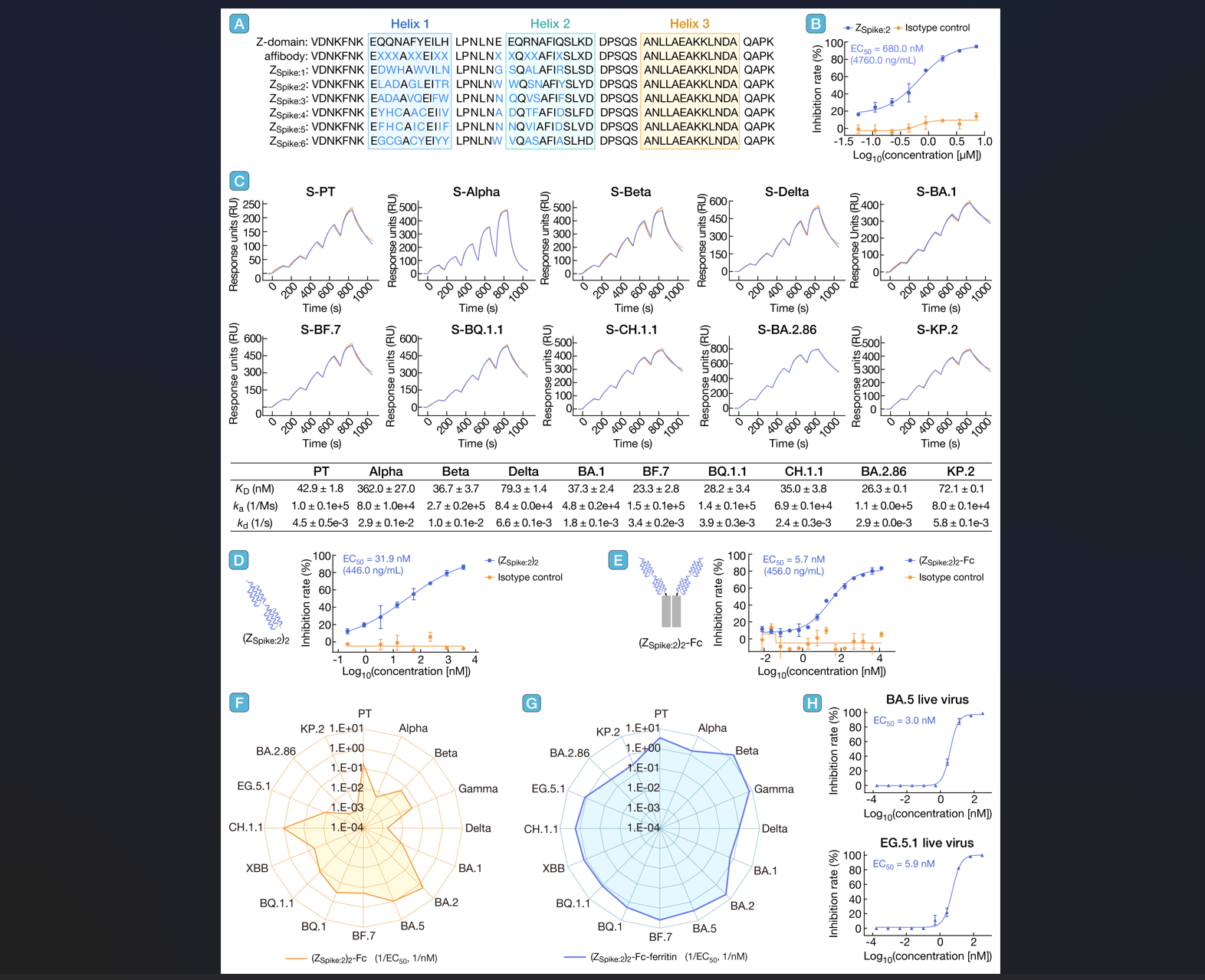
引用:
Yang J, Wang M, Chen Z, et al. Selection and engineering of broad-spectrum antiviral affibody peptides against SARS-CoV-2 variants. hLife 2025; 3: 448–451.
通讯作者:夏朋延

We found that palmitic acid esters of hydroxy stearic acid (PAHSA) can inhibit the immune response caused by SARS-CoV-2. PAHSA enters the cell cytosol and binds to caspase-11, preventing the binding of caspase-11 to LPS, thereby inhibiting the activation of non-canonical inflammasomes and ultimately reducing the serum levels of inflammatory cytokines caused by SARS-CoV-2 infection. The regulation of LPS in cells is currently at the forefront of research. Our previous study has shown that ATGL can bind to LPS and specifically degrade the lipid chains of LPS. This prompted us to consider whether ATGL-synthesized PAHSA also plays a role in non-canonical inflammasome. PAHSA has branched lipid chains similar to the lipid A portion of LPS, which may be the reason for binding to caspase-11. However, the structural basis of PAHSA binding to caspase-11 remains to be clarified, and whether other FAHFAs have the same role needs to be further explored. It is now recognized that hyperinflammation caused by inflammasome plays an important role in the pathophysiology of COVID-19 patients. Most of the previous studies focused on SARS-CoV-2 activating of NLRP3 canonical inflammasome. However, we found that inhibiting caspase-11 in immune cells can also alleviate the symptoms of SARS-CoV-2 infection. This suggests that overactivation of non-canonical pathways may also be an important driver of COVID-19 pathogenesis, which will provide new guidance for future diagnosis and treatment of COVID-19.
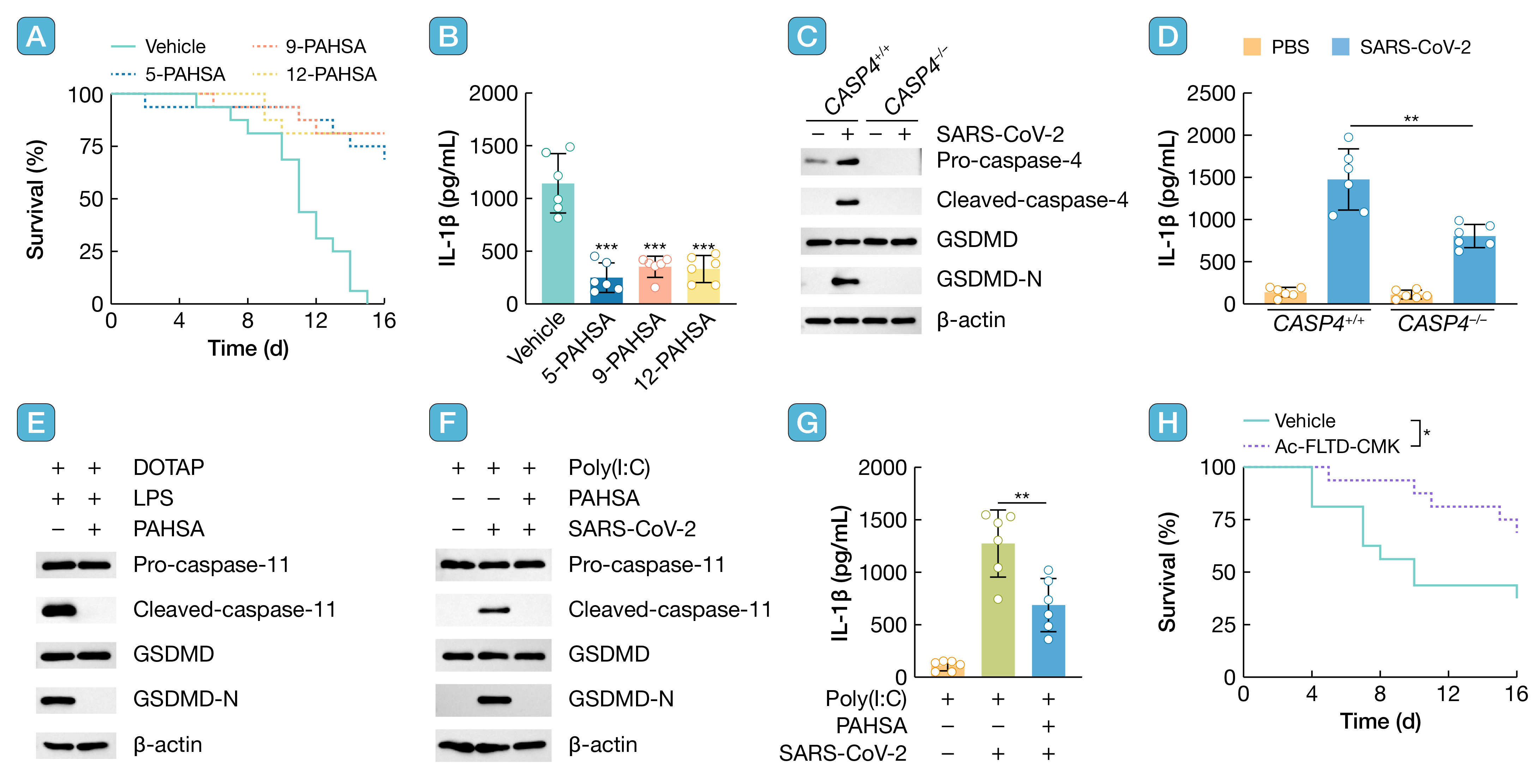
引用:
Kong C, Li Y, Qian Y, et al. Palmitic acid esters of hydroxy stearic acids suppress SARS-CoV-2 infection through inhibiting the non-canonical inflammasome. hLife 2025; 3: 452–454.
通讯作者:David Musoke

After the discovery and approval of coronavirus disease 2019 (COVID-19) vaccines, the World Health Organization (WHO) set global vaccination coverage targets to achieve herd immunity, aiming to vaccinate at least 70% of the global population. However, the availability of COVID-19 vaccination services in Africa was delayed compared to other regions, leading to lower uptake during the initial stages of the pandemic, hence leaving a considerable portion of the population unvaccinated. Despite numerous efforts of the Ministry of Health in Uganda to control COVID-19, gaps remained in addressing vaccine hesitancy, particularly in urban setting. A package of interventions was therefore implemented to increase the uptake and demand for COVID-19 vaccination services, as well as to support the integration of COVID-19 vaccination into routine primary health care (PHC) services.
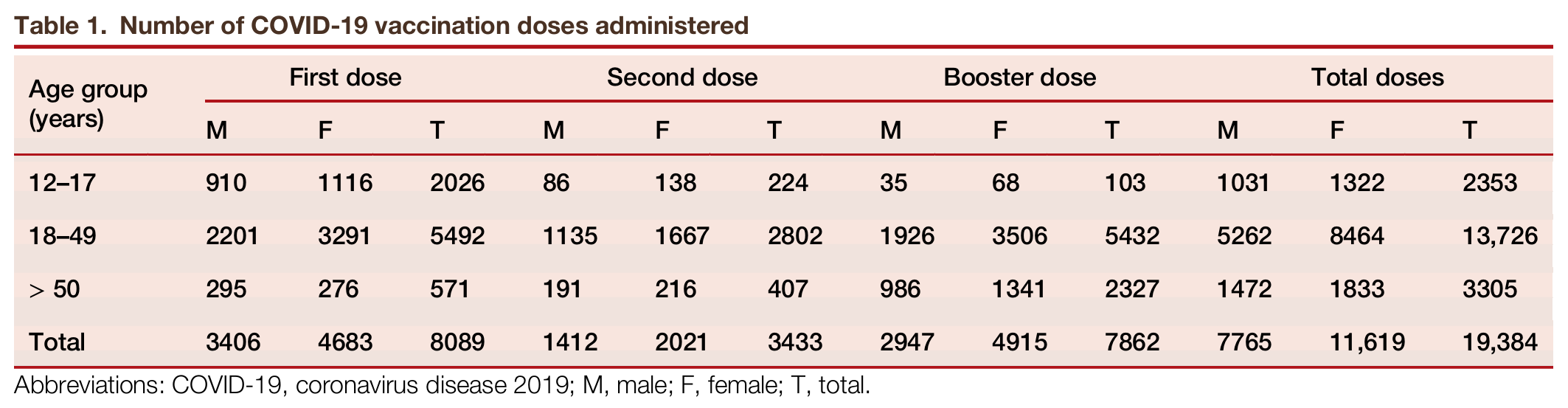
引用:
Musoke D, Masengere P, Ssembuusi A, et al. Addressing COVID-19 vaccination hesitancy through community engagement and integration with primary health care services in Wakiso district, Uganda. hLife 2025; 3: 455–457.
期刊简介
hLife 由高福院士、董晨院士和Jules A. Hoffmann教授(2011诺奖获得者)领衔,是中国科学院微生物研究所主办,中国生物工程学会,浙江大学陈廷骅大健康学院,西湖大学医学院,上海市免疫治疗创新研究院和广州霍夫曼免疫研究所联合支持,与国际出版商爱思唯尔合作的健康科学领域综合性英文期刊。
hLife 聚焦健康科学领域的前沿进展,旨在促进基础研究与临床应用的融合发展。期刊发表与医学相关各研究领域最新成果,学科领域包括(但不限于)病原生物学、流行病学、生理学、免疫学、结构生物学、疾病监测、肿瘤、药物、疫苗和健康政策等。
hLife是一本金色开放获取期刊,月刊出版;2022年成功入选“中国科技期刊卓越行动计划高起点新刊”;2023年11月正式创刊;2024年5月被DOAJ收录;2024年8月被Scopus收录;2024年10月入选“首都科技期刊卓越行动计划——重点英文科技期刊支持项目”;2025年6月入选北京市科委“2025年度支持高水平国际科技期刊建设-强刊提升”项目;2025年8月入选中国科学引文数据库(CSCD)核心库。
hLife实行高标准与高效率并重的同行评审机制:
投稿至给出“是否送审”决定⏰1天
投稿至给出“首轮审稿”决定⏰28天
投稿至给出“是否录用”决定⏰61天
2026年前hLife接收的稿件免收文章处理费(APC)。
https://www.sciencedirect.com/journal/hlife
https://blog.sciencenet.cn/blog-3552961-1507958.html
上一篇:[转载]hLife collection | Vaccines
下一篇:[转载]hLife collection | Organoids