博文
Gut :重庆医科大学发现克服MASH相关肝癌免疫治疗耐药的新策略
|
代谢功能障碍相关脂肪性肝炎驱动的肝癌(Metabolic dysfunction-associated steatohepatitis-related hepatocellular carcinoma, MASH-HCC)是全球增长最快的肝癌亚型,其对免疫检查点抑制剂(如 PD-1/PD-L1 单抗)反应不佳一直是临床治疗面临的重大挑战。与病毒性肝炎(如乙型肝炎病毒HBV)相关肝癌相比,MASH-HCC具有独特的代谢特征与免疫微环境。既往研究提示,糖异生途径关键酶(PCK1、FBP1等)在MASH发生发展中扮演重要角色。然而,肝细胞代谢重编程如何直接调控肿瘤微环境中免疫细胞的功能,进而驱动MASH-HCC进展并导致免疫治疗抵抗,其机制仍不明确。

2025年12月17日,重庆医科大学科研团队在Gut期刊在线发表了题为“PCK1 deficiency promotes MASH-HCC progression by 12-HETE-induced CD8+ T cell dysfunction”的研究论文。 该研究揭示了肝细胞中磷酸烯醇式丙酮酸羧激酶1(phosphoenolpyruvate carboxykinase 1, PCK1/PEPCK1)作为MASH-HCC“代谢检查点”的核心作用,阐明了其通过代谢物12-羟基二十碳四烯酸(12-HETE)直接抑制CD8+T细胞抗肿瘤功能的新机制,为克服MASH-HCC免疫治疗抵抗提供了新的潜在靶点。重庆医科大学感染性疾病分子生物学教育部重点实验室唐霓教授和黄爱龙教授、基础医学院金艾顺教授以及附属第一医院李小松教授为该论文的共同通讯作者;博士后伍康、李罗、刘异和汪凯研究员为该论文的共同第一作者。
为了探究肝脏代谢重编程对MASH-HCC的影响,研究团队首先通过多数据集联合分析发现,糖异生途径限速酶PCK1在MASH-HCC中显著低表达。这一现象在MASH-HCC临床样本和小鼠模型中得到验证,提示PCK1可能对MASH-HCC起保护作用。进一步通过单细胞转录组测序与多色免疫荧光分析发现,肝细胞PCK1缺失导致肿瘤组织中CD8+T细胞功能障碍;而清除CD8+T细胞则逆转了PCK1过表达对MASH-HCC的保护作用,表明PCK1是通过增强CD8+T细胞功能来抑制MASH-HCC进展。
那么,PCK1缺失的肿瘤细胞如何调控CD8+T细胞功能?研究人员通过非靶标代谢组学等实验发现,PCK1缺失通过升高活性氧(ROS)增强了ALOX15酶活性,促进了花生四烯酸途径代谢物12-HETE大量产生。该代谢物被CD8+T细胞摄取,并直接结合转录因子BACH1,激活p38/MAPK信号通路,进而抑制CD8+T细胞效应功能。最终,在MASH-HCC小鼠模型中,过表达肝细胞PCK1或抑制12-HETE的产生,均可重塑肿瘤免疫微环境、增强CD8+T细胞抗肿瘤功能,并显著提高免疫检查点抑制剂疗效。
综上所述,该研究聚焦代谢失调相关脂肪性肝炎相关肝癌免疫治应答不佳这一临床痛点,首次提出肝细胞代谢酶PCK1通过代谢物12-HETE直接调控T细胞功能的新范式,揭示了12-HETE以“配体-转录因子”直接互作的非经典方式抑制CD8+T细胞效应功能,拓展了代谢-免疫调控的理论认知。基于此,研究团队提出靶向“PCK1-12-HETE”代谢轴以克服免疫治疗抵抗的新策略,为脂肪肝相关肝癌的精准联合疗法提供了重要分子靶点与干预思路。
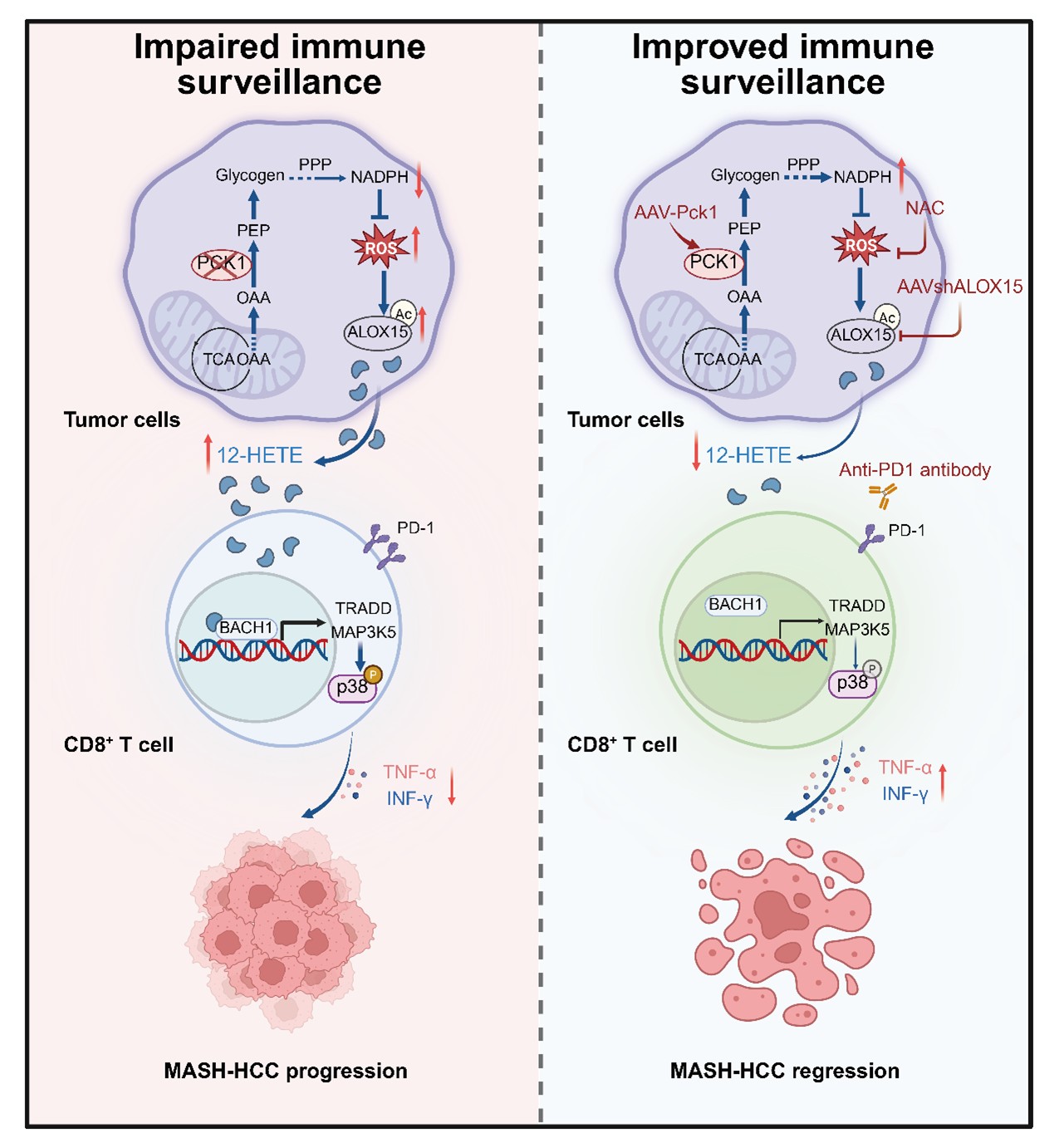
肝细胞PCK1缺失导致代谢物12-HETE抑制CD8+T细胞功能促进MASH-HCC进展模式图
摘要:Abstract
Background Metabolic dysfunction-associated steatohepatitis-related hepatocellular carcinoma (MASH-HCC) has been reported to be less responsive to immune checkpoint inhibitors, which may be associated with metabolic reprogramming of tumour cells and abnormal tumour microenvironment.
Objective Here, we aim to investigate the role of gluconeogenic enzyme phosphoenolpyruvate carboxykinase 1 (PCK1) in MASH-HCC and its interplay with the tumour microenvironment.
Design Hepatocyte-specific phosphatase and tensin homologue (Pten) and Pck1 biallelic knockout mice were established to induce MASH-HCC. Single-cell RNA sequencing and multiparametrical flow cytometry were performed to analyse the immune landscape alterations. Untargeted metabolomics was conducted to elucidate the hepatic metabolism dysregulation.
Results PCK1 is downregulated in tumour tissues compared with adjacent non-cancerous tissues from patients with MASH-HCC. Hepatocyte-specific Pck1 knockout mice exhibited markedly increased tumorigenesis in dietary models and genetic models of spontaneous MASH-HCC, together with inhibited effector function of tumour-infiltrating CD8+ T cells. Mechanistically, PCK1 deficiency induces the accumulation of endogenous metabolite 12-hydroxyeicosatetraenoic acid (12-HETE), which can be taken up by CD8+ T cells and activate the p38 mitogen-activated protein kinase pathway by directly interacting with the BTB and CNC homology 1 transcription factor, ultimately leading to CD8+ T cells dysfunction. Notably, PCK1 restoration or 12-HETE inhibition combined with anti-PD-1 treatment increases the antitumour capability of CD8+ T cells and suppresses MASH-HCC development.
Conclusion This study reveals the pivotal role of the hepatic cell-intrinsic enzyme PCK1 in mediating CD8+ T cell dysfunction via 12-HETE-p38 signalling in MASH-HCC. PCK1 could be a metabolic checkpoint to enhance the efficacy of anti-PD-1 immunotherapy in MASH-HCC.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Metabolic dysfunction-associated steatohepatitis-related hepatocellular carcinoma (MASH-HCC) exhibits distinct metabolic and immune characteristics.
Human patients and murine models of MASH-HCC responded poorly to immune checkpoint inhibitors.
Phosphoenolpyruvate carboxykinase 1 (PCK1) has been reported as a tumour suppressor gene in HCC and has shown a protective role in metabolic-associated fatty liver disease.
WHAT THIS STUDY ADDS
Hepatic PCK1 deficiency promotes MASH-HCC development by reshaping the tumour microenvironment.
Hepatocyte-specific PCK1 restoration inhibits MASH-HCC progression via enhancing the antitumour activity of CD8+ T cells.
Loss of PCK1 promotes the production of 12-hydroxyeicosatetraenoic acid (12-HETE), which is responsible for inducing CD8+ T cells dysfunction in MASH-HCC.
Inhibition of 12-HETE accumulation improves the efficiency of anti-PD-1 therapy in MASH-HCC mouse models.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Targeting the PCK1-12-HETE metabolic pathway may provide a potential therapeutic strategy to enhance the efficacy of immune checkpoint inhibitors in MASH-HCC.
原文链接:https://doi.org/10.1136/gutjnl-2024-334562
参考文献:
1 Yahoo N, Dudek M, Knolle P, et al. Role of immune responses in the development of NAFLD-associated liver cancer and prospects for therapeutic modulation. J Hepatol. 2023;79(2):538-551. doi:10.1016/j.jhep.2023.02.033
2 Wang X, Zhang L, Dong B. Molecular mechanisms in MASLD/MASH-related HCC. Hepatology. 2025;82(5):1303-1324. doi:10.1097/HEP.0000000000000786
3 Pfister D, Núñez NG, Pinyol R, et al.NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450-456. doi:10.1038/s41586-021-03362-0
4 Leslie J, Mackey JBG, Jamieson T, et al.CXCR2 inhibition enables NASH-HCC immunotherapy. Gut. 2022;71(10):2093-106. doi: 10.1136/gutjnl-2021-326259
https://blog.sciencenet.cn/blog-446272-1514957.html
上一篇:[转载]氨基己糖合成途径通过免疫检查点翻译延伸驱动肿瘤免疫逃逸新机制
下一篇:[转载]hLife:中国科学院钟劲团队揭示O-GlcNAc糖基化调控外泌体释放新机制