博文
科学家揭示了硒蛋白的力量  精选
精选
||
科学家揭示了硒蛋白的力量
诸平
据日本大阪大学(Osaka University)2025年2月13日提供的消息,健康衰老的秘诀吗?科学家揭示了硒蛋白的力量(The Secret to Healthy Aging? Scientists Uncover the Power of Selenoproteins)。
日本大阪大学的一个研究小组调查了抗氧化酶(antioxidant enzymes)对特定细胞类型的影响及其在与年龄有关的疾病中的作用。
许多食品都标榜其抗氧化功效,有助于中和活性氧(reactive oxygen species简称ROS)这种高活性分子,这种分子会破坏人体细胞中的脂质、蛋白质和DNA。过量的ROS积累与年龄相关的疾病(包括癌症)有关,这突出了维持氧化/抗氧化系统平衡的必要性。大阪大学和日本其他机构的研究人员,2025年1月7日已经在《血液》(Blood)杂志上发表了一项研究,探讨了硒蛋白的关键抗氧化作用。原文详见:Yumi Aoyama, Hiromi Yamazaki, Koutarou Nishimua, Masaki Nomura, Tsukasa Shigehiro, Takafumi Suzuki, Weijia Zang, Yota Tatara, Hiromi Ito, Yasutaka Hayashi, Yui Koike, Miki Fukumoto, Atsushi Tanaka, Yifan Zhang, Wataru Saika, Chihiro Hasegawa, Shuya Kasai, Yingyi Kong, Yohei Minakuchi, Ken Itoh, Professor, Masayuki Yamamoto, Shinya Toyokuni, Atsushi Toyoda, Tomokatsu Ikawa, Akifumi Takaori-Kondo, Daichi Inoue. Selenoprotein-Mediated Redox Regulation Shapes the Cell Fate of HSCs and Mature Lineages. Blood, 7 January 2025, DOI: 10.1182/blood.2024025402
参与此项研究的除了来自日本大阪大学(Graduate School of Medicine and Frontier Biosciences, Osaka University, Japan)的研究人员之外,还有来自日本京都大学(Graduate School of Medicine, Kyoto University, Japan)、日本CiRA基金会(Facility for iPS Cell Therapy, CiRA Foundation, Japan)、日本千叶的东京理科大学(Tokyo University of Science, Chiba, Japan)、日本东北大学{Tohoku University, Tohoku Medical Megabank Organization, Sendai, Japan; Advanced Research Center for Innovations in Next-Generation Medicine (INGEM), Tohoku University, Japan}、日本广崎大学(Biomedical Research Center, Hirosaki University Graduate School of Medicine, Hirosaki, Japan)、日本神户市生物医学研究与创新基金会生物医学研究与创新研究所(Institute of Biomedical Research and Innovation, Foundation for Biomedical Research and Innovation at Kobe, Kobe, Japan)、日本东京医科牙科大学(Medical Research Insitute, Tokyo Medical and Dental University, Japan)、日本京都桂病院(Kyoto-Katsura Hospital, Japan)、日本志贺医学科学大学(Shiga University of Medical Science, Japan)、日本名古屋大学(Nagoya University Graduate School of Medicine, Nagoya, Japan; Center for Low-temperature Plasma Sciences, Nagoya University, Japan)以及日本国家遗传学研究所(National Institute of Genetics, Mishima, Japan)的研究人员。该研究考察了破坏硒蛋白的产生如何影响不同的细胞类型和造血,即血细胞形成的过程。
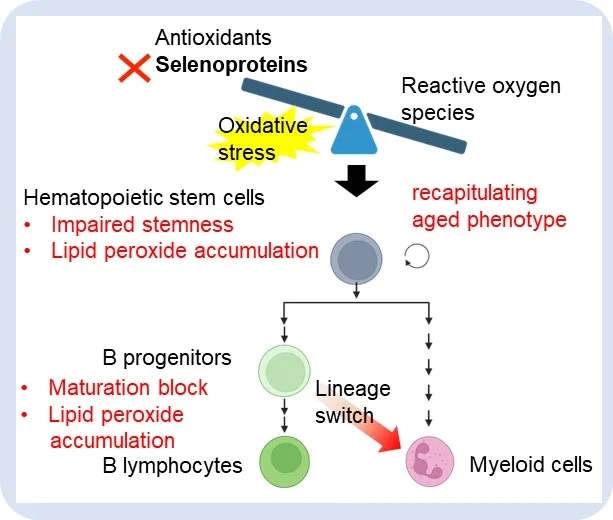
人体细胞有25种不同的硒蛋白。这些抗氧化酶有助于将危险的活性氧(如脂质过氧化物)转化为更安全的形式。脂质过氧化物的积累可以影响被称为造血干细胞(hematopoietic stem cells简称HSCs)的关键细胞,这是一种在衰老疾病中观察到的现象。
“我们观察到衰老的造血干细胞经常显示硒蛋白合成受损,但尚不清楚这是如何导致细胞衰老的,以及它是否可以逆转,”该研究的联合首席作者青山裕美(Yumi Aoyama)说。“我们假设硒蛋白是抗氧化系统的关键部分,可以对抗HSCs中与年龄相关的变化。”
研究硒蛋白破坏的影响(Investigating the Effects of Selenoprotein Disruption)
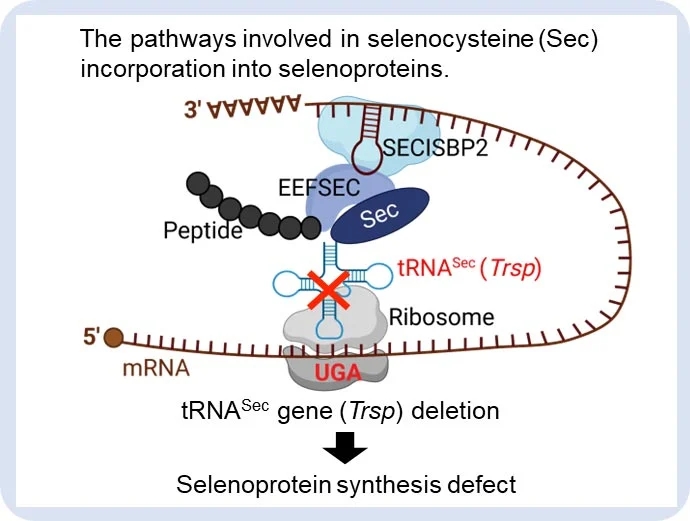
为了研究这个问题,研究小组使用了一个老鼠模型,该模型中有一个特定的基因被敲除,导致硒蛋白的产生被破坏。然后,他们研究了这对不同细胞类型的影响,发现基因敲除对造血干细胞和B细胞谱系的免疫细胞(白细胞类型)产生负面影响,但对骨髓细胞(不同的免疫细胞家族)几乎没有影响。
该研究的另一位主要作者山嵜博未(Hiromi Yamazaki)解释说:“基因敲除最显著的结果包括B淋巴细胞减少症,这意味着B细胞比预期的要少。造血干细胞(HSCs)也具有有限的自我更新能力。”
硒蛋白缺乏与衰老相关变化的联系(Linking Selenoprotein Deficiency to Aging-Related Changes)
这些观察结果,以及这些细胞类型中衰老相关基因表达水平的增加,与在年龄相关疾病中常见的情况一致。进一步的研究表明,这种作用是由脂质过氧化作用驱动的。此外,小鼠模型细胞的实验显示,硒蛋白合成的破坏可能支持B祖细胞(B progenitors)向髓系细胞家族(myeloid cell family)的转变。
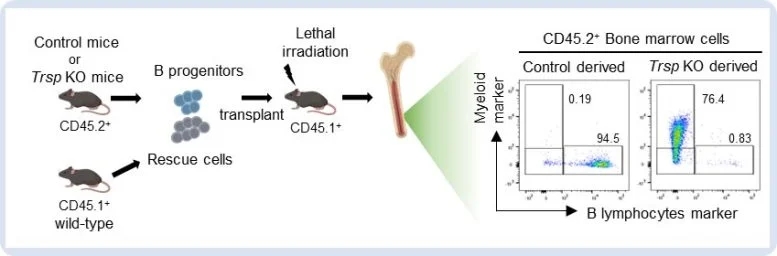
Fig. 4 Transplantation experiment of B progenitors from CD45.2+ control or Trsp KO mice into lethally irradiated CD45.1+ recipients. Trsp KO B progenitors demonstrated a significant potential to generate myeloid cells in the recipient bone marrow.(Created in BioRender. Yamazaki, H. (2025) https://BioRender.com/ y92h928). Credit: Yumi Aoyama
该研究的资深作者井上大地(Daichi Inoue)说:“我们的数据表明,当硒蛋白的保护作用丧失时,会产生明显的谱系特异性影响。这些酶对对抗衰老过程中积累的脂质过氧化物至关重要。”
研究人员还通过对敲除小鼠的喂养实验研究了造血机制。他们发现,膳食维生素E可以保护造血功能,并具有修复受损B细胞分化的能力。
这项研究显示了硒蛋白的抗氧化功能,以及它们如何确保适当的HSC自我更新和B细胞谱系免疫细胞成熟。由于基因敲除小鼠表现出与衰老正常小鼠相似的表型,研究结果表明,解决硒蛋白产生相关问题可能有助于对抗与年龄相关的疾病。
本研究得到了日本科学促进会(Japan Society for the Promotion of Science)、日本医学研究和开发局(Japan Agency for Medical Research and Development)、日本科学技术振兴机构( Japan Science and Technology Agency)、内藤基金会(Naito Foundation)、美国血液学学会(American Society of Hematology)、日本血液学学会(Japanese Society of Hematology)、小野医学研究基金会(Ono Medical Research Foundation)、小野肿瘤制药基金会(Ono Pharmaceutical Foundation for Oncology)、三菱基金会(Mitsubishi Foundation)、细胞科学研究基金会(Cell Science Research Foundation)、小林癌症研究基金会(Kobayashi Foundation for Cancer Research)、武田科学基金会(Takeda Science Foundation)、中盖创新药物发现科学基金会(Chugai Foundation for Innovative Drug Discovery Science)、癌症研究促进基金会(Foundation for Promotion of Cancer Research)、白血病之友研究基金(Friends of Leukemia Research Fund)、高松公主癌症研究基金(Princess Takamatsu Cancer Research Fund)、望田医学和药物研究纪念基金会(Mochida Memorial Foundation for Medical and Pharmaceutical Research)、MSD生命科学基金会(MSD Life Science Foundation)和公共利益联合基金会(Public Interest Incorporated Foundation)、SENSHIN医学研究基金会(SENSHIN Medical Research Foundation)的资助。
上述介绍仅供参考,欲了解更多信息敬请注意浏览原文和相关报道。
Selenoproteins: The fountain of youth? | EurekAlert!
l Disruption of antioxidant selenoprotein synthesis exhibited significant defects in B lymphocyte development and HSC function.
l Cell context and lineage dictate sensitivity to selenoprotein synthesis defects, with lipid peroxidation and ferroptosis serving as drivers.
The maintenance of cellular redox balance is crucial for cell survival and homeostasis and is disrupted with aging. Selenoproteins, comprising essential antioxidant enzymes, raise intriguing questions about their involvement in hematopoietic aging and potential reversibility. Motivated by our observation of mRNA downregulation of key antioxidant selenoproteins in aged human hematopoietic stem cells (HSCs) and previous findings of increased lipid peroxidation in aged hematopoiesis, we employed tRNASec gene (Trsp) knockout (KO) mouse model to simulate disrupted selenoprotein synthesis. This revealed insights into the protective roles of selenoproteins in preserving HSC stemness and B-lineage maturation, despite negligible effects on myeloid cells. Notably, Trsp KO exhibited B lymphocytopenia and reduced HSCs' self-renewal capacity, recapitulating certain aspects of aged phenotypes, along with the upregulation of aging-related genes in both HSCs and pre-B cells. While Trsp KO activated an antioxidant response transcription factor NRF2, we delineated a lineage-dependent phenotype driven by lipid peroxidation, which was exacerbated with aging yet ameliorated by ferroptosis inhibitors such as vitamin E. Interestingly, the myeloid genes were ectopically expressed in pre-B cells of Trsp KO mice, and KO pro-B/pre-B cells displayed differentiation potential toward functional CD11b+ fraction in the transplant model, suggesting that disrupted selenoprotein synthesis induces the potential of B-to-myeloid switch. Given the similarities between the KO model and aged wild-type mice, including ferroptosis vulnerability, impaired HSC self-renewal and B-lineage maturation, and characteristic lineage switch, our findings underscore the critical role of selenoprotein-mediated redox regulation in maintaining balanced hematopoiesis and suggest the preventive potential of selenoproteins against aging-related alterations.
https://blog.sciencenet.cn/blog-212210-1473113.html
上一篇:新研究引起人们关注:“好胆固醇”可能对眼睛有害
下一篇:新的人源化小鼠使科学家更接近逆转衰老