博文
将太阳能储存在分子中并将其转化为热能  精选
精选
||
将太阳能储存在分子中并将其转化为热能
诸平
据法国国家科学研究中心{Centre national de la recherche scientifique (CNRS)}2024年9月25日提供的消息,将太阳能储存在分子中并将其转化为热能(Storing solar energy in molecules and converting it into heat energy)。
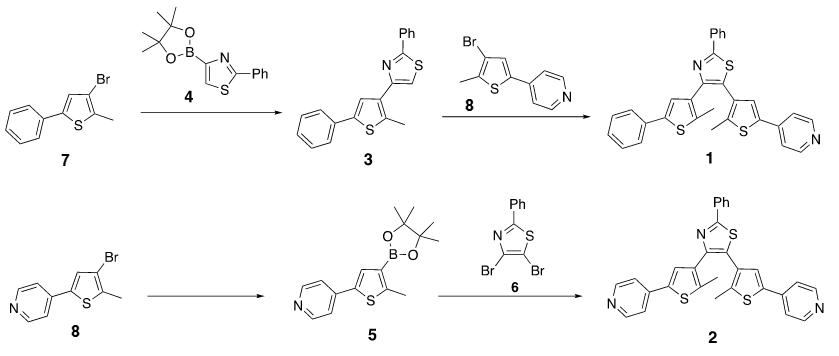
Figure S1: Syntheses of terarylenes 1 and 2.
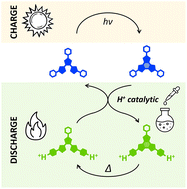
光致变色分子(Photochromic molecules)可以储存光能,但它们能将光能转化为热能吗?这是法国国家科学研究中心{Centre national de la recherche scientifique (CNRS)}和巴黎高等师范学院(ENS Paris-Saclay)的科学家们最近遇到的挑战。该团队确定了一种机制,通过这种机制,这些分子以热形式释放能量,随后可以在多个能量存储/转换循环中重复使用。
由于添加了极少量的酸,化学家们能够有效地控制这个可逆过程中的热反反应(thermal back reaction)。这些分子非常耐光,是原始光敏开关家族的一部分,它们以化学形式存储太阳能,然后“按需”将其转化为热能。
这项研究2024年9月25日在《化学科学》(Chemical Science)杂志网站发表——Léa Chocron, Nicolò Baggi, Enrique Ribeiro, Vincent Goetz, Pei Yu, Keitaro Nakatani, Rémi Métivier. Acid-sensitive photoswitches: towards catalytic on-demand release of stored light energy. Chemical Science, First published: 25 Sep 2024. https://pubs.rsc.org/en/content/articlelanding/2024/sc/d4sc04973j
此研究为开发高性能、可控的可再生能源存储系统铺平了道路。
参与此项研究的有来自法国巴黎萨雷大学、巴黎高师、法国国家科研中心共建的超分子与大分子光物理与光化学实验室{Université Paris-Saclay, ENS Paris-Saclay, CNRS, Le laboratoire de Photophysique et Photochimie Supramoléculaires et Macromoléculaires (PPSM), 91190 Gif-sur-Yvette, France};法国巴黎萨雷大学、法国国家科研中心共建的奥尔赛分子化学与材料研究所{Université Paris-Saclay, CNRS, Institute of Molecular Chemistry and Materials of Orsay (ICMMO), 91400 Orsay, France}以及法国国家科研中心工艺、材料及太阳能实验室(CNRS, Laboratoire PROcédés Matériaux et Energie Solaire (PROMES), 66100 Perpignan, France)的研究人员。
本研究的实验工作在超分子和大分子光物理和光化学实验室{PPSM}与奥赛分子化学和材料研究所{ICMMO}的团队合作,此外还有法国国家科研中心和佩皮尼昂大学合建的工艺、材料和太阳能实验室(PROMES)的研究人员参与。得到法国国家研究机构{Agence Nationale de la Recherche (ANR-17-CE07-0056-01 and ANR-21-CE50-0025-02)}和巴黎-萨克雷物理化学联合会(Fédération de Chimie Physique de Paris-Saclay)的资助,也得到了法国高等教育和研究部博士奖学金(PhD fellowship from the French Ministry of Higher Education and Research)的支持。
上述介绍,仅供参考。欲了解更多信息,敬请注意浏览原文或者相关报道。
Photochromic compounds are promising for a variety of applications, including molecular solar thermal (MOST) energy storage. The energy release step and cyclability are critical issues to be addressed for the development of this technology. We report herein the synthesis and characterization of two diarylethene molecules featuring one (1) or two (2) pyridine groups as protonatable moieties. Upon UV irradiation, both molecules undergo a cyclization reaction from the open form (OF) to the closed form (CF). Both CF are stable for a few days in acetonitrile, and the addition of acid leads to a 600 (1) or 1500-fold (2) acceleration of the ring-opening reaction, even in catalytic amounts. A kinetic model is proposed to simulate the reaction, elucidating the contribution of each step to the kinetics and evidencing the importance of the kinetic control over the protonation thermodynamic equilibrium. Data fitting leads to the rates of elementary steps and turnover numbers (TON). Following a complete reaction cycle, neutralization of the acid by an equivalent amount of base allowed further cycles. This study represents a significant advancement in the cyclability and the control of the on-demand triggering of the energy-releasing ring-opening reaction of diarylethenes for future MOST applications.
https://blog.sciencenet.cn/blog-212210-1452828.html
上一篇:革命性的抗衰老疗法可以延长寿命25岁
下一篇:垂直农业如何改变食物生产