博文
ABBS: D-amino acid substitution of antimicrobial peptide
|||
D-amino acid substitution enhances the stability of antimicrobial peptide polybia-CP
Fengjing Jia, Jiayi Wang, Jinxiu Peng, Ping Zhao, Ziqing Kong, Kairong Wang, Wenjin Yan, and Rui Wang
Institute of Biochemistry and Molecular Biology, School of Life Sciences, Key Laboratory of Preclinical Study for New Drugs of Gansu Province, School of Basic Medical Sciences, Lanzhou University, Lanzhou 730000, China
Acta Biochim Biophys Sin 2017, 49: 916–925; doi: 10.1093/abbs/gmx091
With the increasing emergence of resistant microbes toward conventional antimicrobial agents, there is an urgent need for the development of antimicrobial agents with novel action mode. Antimicrobial peptides (AMPs) are believed to be one kind of ideal alternatives. However, AMPs can be easily degraded by protease, which limited their therapeutic use. In the present study, D-amino acid substitution strategy was employed to enhance the stability of polybia-CP. We investigated the stability of peptides against the degradation of trypsin and chymotrypsin by determining the antimicrobial activity or determining the HPLC profile of peptides after incubation with proteases. Our results showed that both the all D-amino acid derivative (D-CP) and partial D-lysine substitution derivative (D-lys-CP) have an improved stability against trypsin and chymotrypsin. Although D-CP takes left-hand α-helical conformation and D-lys-CP loses some α-helical content, both of the D-amino acid-substituted derivatives maintain their parental peptides’ membrane active action mode. In addition, D-lys-CP showed a slight weaker antimicrobial activity than polybia-CP, but the hemolytic activity decreased greatly. These results suggest that D-CP and D-lys-CP can offer strategy to improve the property of AMPs and may be leading compounds for the development of novel antimicrobial agents.
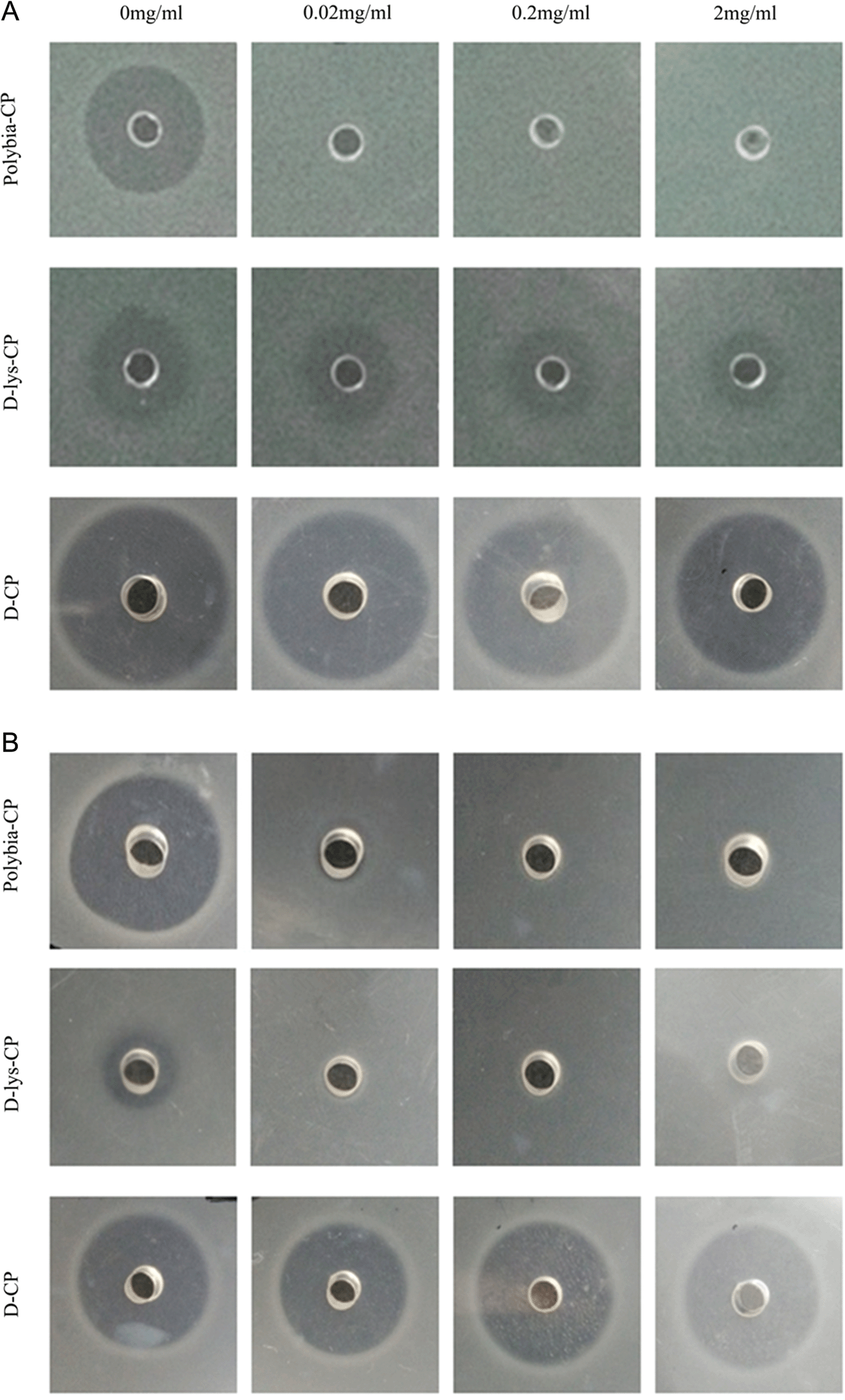
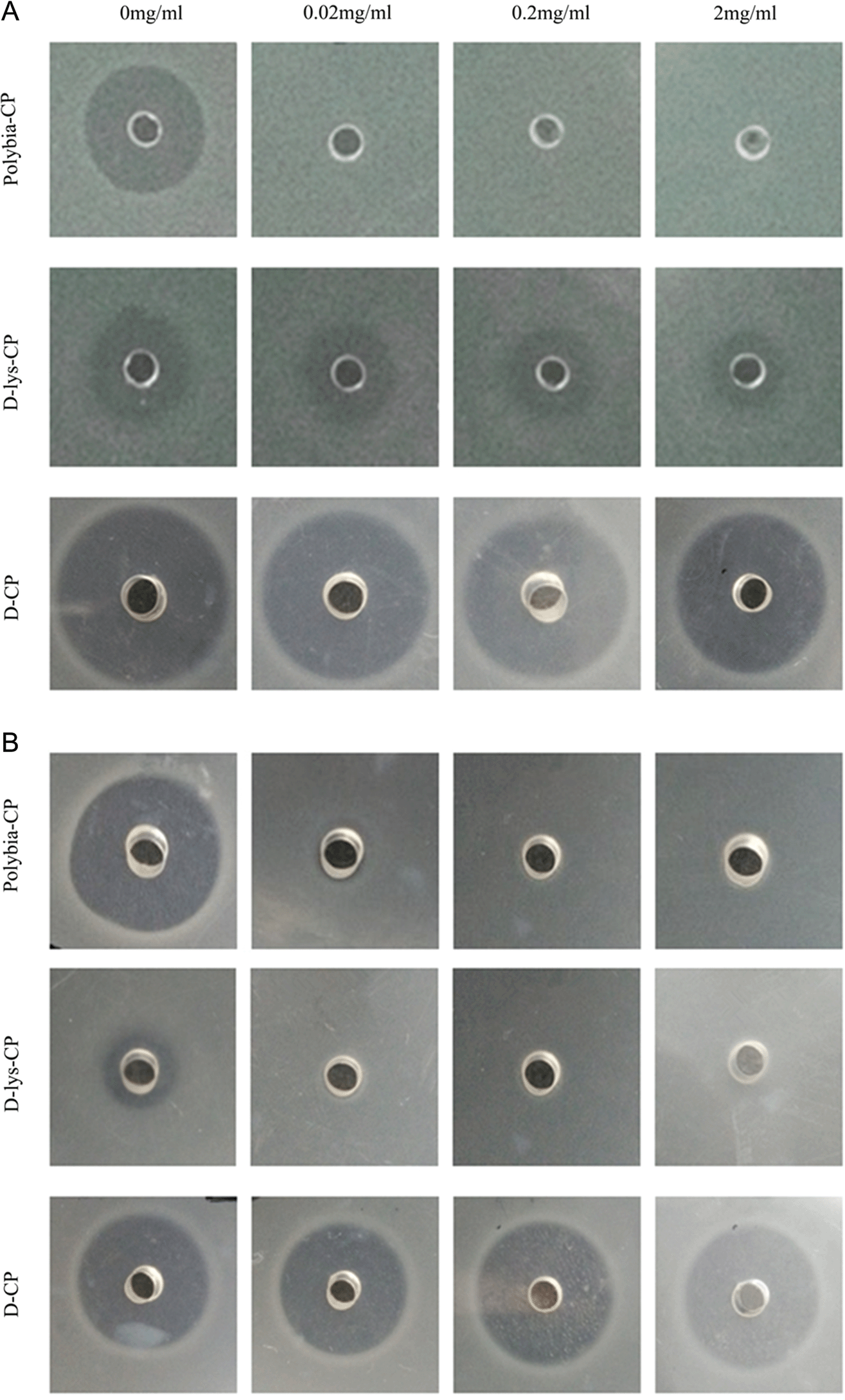
Protease on the bactericidal activity of polybia-CP, D-lys-CP, and D-CP
阅读原文: http://www.abbs.org.cn/arts.asp?id=4215
获取全文: abbs@sibs.ac.cn
相关论文:
1 Computational Design of Thermostabilizing D-Amino Acid Substitutions
2 Structure-antimicrobial activity relationship between pleurocidin and its enantiomer
3 Initiating translation with D-amino acids

https://blog.sciencenet.cn/blog-592748-1180479.html
上一篇:ABBS: HMGB1 and Saquinavir protection on lung injury
下一篇:ABBS: SLC39A7 (Zip7) in human colorectal cancer