博文
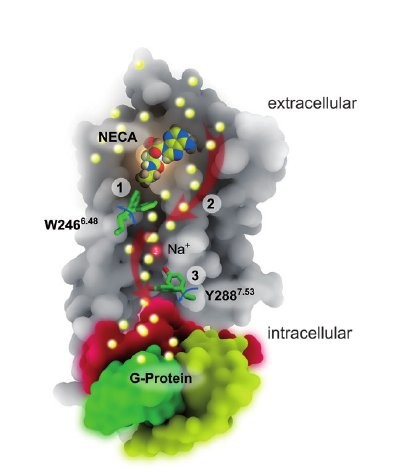
W6.48 Opens a Gate for a Continuous Intrinsic Water Pathway
||
Angew Chem Int Edit . 17/11/2014; 53(1-5). DOI: 10.1002/anie.201409679
https://blog.sciencenet.cn/blog-355217-844399.html
上一篇:Fix NTFS disk error under linux
下一篇:best PDF editor under Linux