博文
Activation of GPCR correlates continuous water pathway
|||
Activation of G-protein-coupled receptors correlates with the formation of a continuous internal water pathway.
Shuguang Yuan, Slawomir Filipek, Krzysztof Palczewski, Horst Vogel
Nature Communication doi:10.1038/ncomms5733
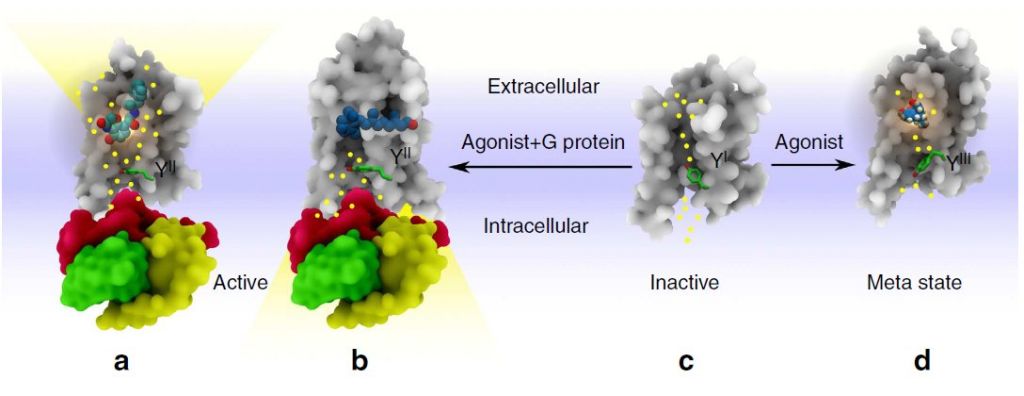
A schematic view of three different rotamer conformations of the highly conserved Y7.53 residue. (a) The YII conformation is preferred in activated GPCRs; during formation it is accessed by extracellular water molecules forming a continuous internal water network. (b) For receptors
similar to Rho and S1PR1 the YII state is associated with the cytoplasmic influx of water. GPCRs are shown in grey and the G-protein subunits are displayed in different colours. (c) The YI conformation is preferred in inactive GPCRs with two hydrophobic layers blocking a continuous water pathway. (d) The YIII conformation is preferred in GPCR meta states with one hydrophobic layer located next to the NPxxY motif and blocking formation of a continuous water pathway.
Abstract
Recent crystal structures of G-protein-coupled receptors (GPCRs) have revealed ordered internal water molecules, raising questions about the functional role of those waters for receptor activation that could not be answered by the static structures. Here, we used molecular dynamics simulations to monitor—at atomic and high temporal resolution—conformational changes of central importance for the activation of three prototypical GPCRs with known crystal structures: the adenosine A2A receptor, the β2-adrenergic receptor and rhodopsin. Our simulations reveal that a hydrophobic layer of amino acid residues next to the characteristic NPxxY motif forms a gate that opens to form a continuous water channel only upon receptor activation. The highly conserved tyrosine residue Y7.53 undergoes transitions between three distinct conformations representative of inactive, G-protein activated and GPCR metastates. Additional analysis of the available GPCR crystal structures reveals general principles governing the functional roles of internal waters in GPCRs.
https://blog.sciencenet.cn/blog-355217-826323.html
上一篇:a tool for modified residues topology generation
下一篇:Nobel Prize 2014 in Chemistry announced