博文
祝贺高娟娟、毛梦茜、李培文和刘如梦合作文章在ACS Applied Materials & Interfaces发表
||
Segmentation and Re-encapsulation of Porous PtCu Nanoparticles by Generated Carbon Shell for Enhanced Ethylene-glycol Oxidation and Oxygen-reduction Reaction
Juanjuan Gao †, ‖, 1, Mengxi Mao †, 1, Peiwen Li †, 1, Rumeng Liu †, 1, Haiou Song ‡, *, Kuan Sun §, Shupeng Zhang †, *
1 Juanjuan Gao, Mengxi Mao, Peiwen Li and Rumeng Liu contributed equally to this work.
ABSTRACT
Hierarchical porous carbon-encapsulated ultrasmall PtCu nanoparticles (NPs) (UsPtCu@C) were constructed based on segmentation and re-encapsulation of porous PtCu NPs by using glucose as a green biomass carbon source. The synergistic electronic effect from the bimetallic elements can enhance the catalytic activity by adjusting the surface electronic structure of the Pt. Most importantly, the generated porous carbon shell provided a large contact surface area, excellent electrical conductivity and structural stability, and the ultrasmall PtCu NPs exhibited an increased electrochemical performance compared with their PtCu matrix because of the exposure of more catalytically active centers. This synergistic relationship between the components resulted in an enhanced catalytic activity and a better stability of the obtained UsPtCu@C for ethylene-glycol oxidation reaction and the oxygen-reduction reaction in alkaline electrolyte, which was higher than the PtCu NPs and commercial Pt/C (20 wt% Pt on Vulcan XC-72). The electrochemically active surface areas of the UsPtCu@C, PtCu NPs and commercial Pt/C were calculated to be approximately 230.2, 32.8 and 64.0 m2/gPt, respectively; the mass activity of the UsPtCu@C for the ethylene-glycol oxidation reaction was 8.5 A/mgPt, which was 14.2 and 8.5 times that of PtCu NPs and commercial Pt/C, respectively. The specific activity of UsPtCu@C was 3.7 , which was 2.1 and 2.3 times that of PtCu NPs and commercial Pt/C, respectively. The onset potential (Eon-set) of UsPtCu@C for the oxygen-reduction reaction was 0.96 V (vs. reversible hydrogen electrode, RHE), which was 110 and 60 mV higher than PtCu and commercial Pt/C, respectively. The half-wave potentials (E1/2) of UsPtCu@C, PtCu and Pt/C were 0.88, 0.56 and 0.82 V (vs. RHE), respectively, which indicated that the UsPtCu@C catalyst had an excellent bifunctional electrocatalytic activity.
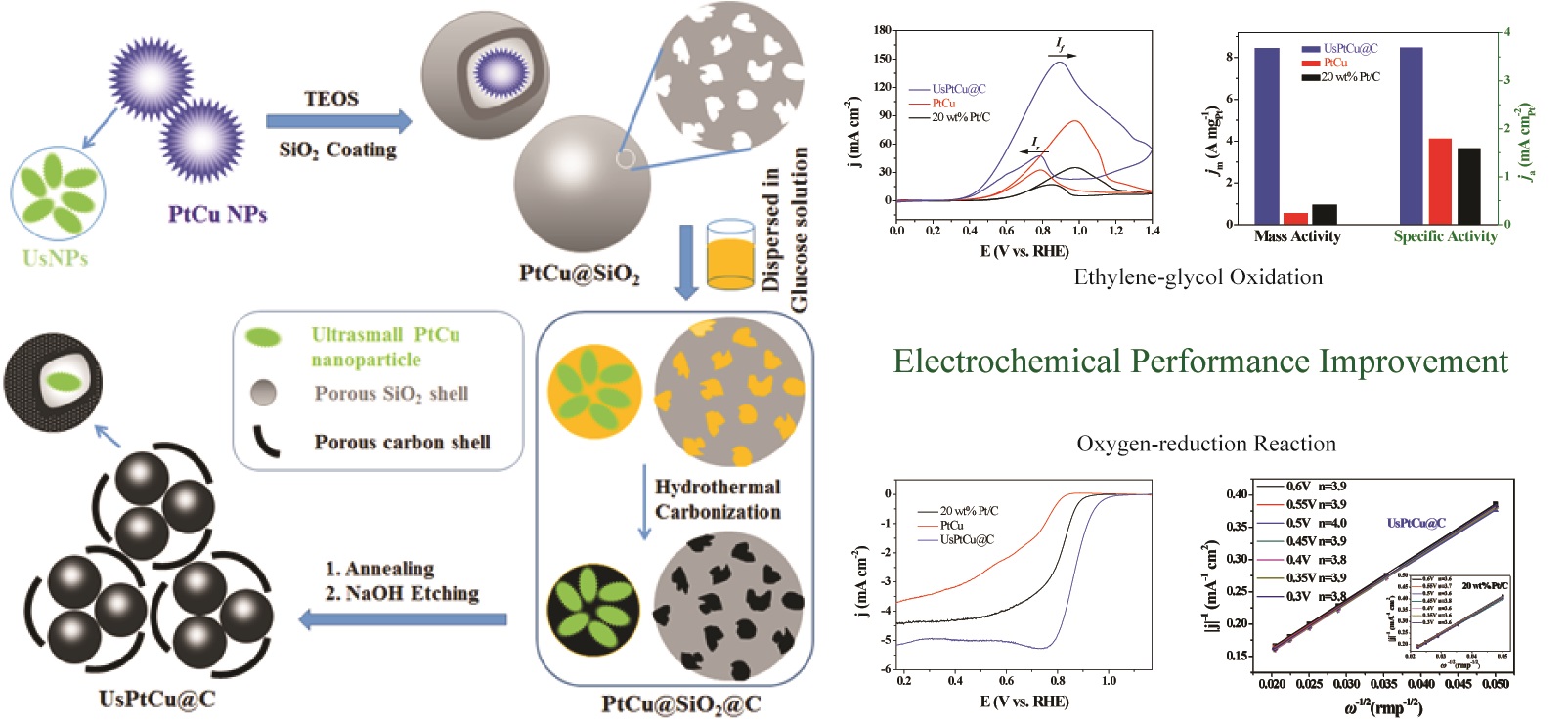
为直接解决能源危机,降低环境污染,在室温下通过简单的超声处理,一步合成了PtCu纳米簇。随后,以葡萄糖为碳源,在SiO2模板存在下通过水热碳化法制得了多孔C包裹的PtCu纳米簇(PtCu@C)。由于无机碳壳具有大的比表面积、良好的导电性和优异的结构稳定性,以及具有多孔结构的双金属PtCu纳米簇作为催化活性中心,所获得的PtCu@C催化剂可以作为双功能催化剂同时用于阳极EGOR和阴极ORR的催化。PtCu@C对EGOR的质量活性为8.5 A/mgPt,分别是PtCu纳米簇和商业Pt/C的14.2和8.5倍;比活性为3.7,分别是PtCu纳米簇和商业Pt/C的2.1和2.3倍。PtCu@C对ORR的Eon-set为0.96 V(相对于RHE),比PtCu和商业Pt/C分别提高了110 mV和60 mV;PtCu@C、PtCu和Pt/C的E1/2分别为0.88、0.56、0.82 V(相对于RHE)。
https://blog.sciencenet.cn/blog-311896-1214144.html
上一篇:祝贺陈多哲等同学研究成果在Electrochimica Acta发表
下一篇:祝贺陈多哲、朱伟青和庞迪三位同学顺利通过硕士毕业答辩